Petroleum is a naturally occurring, toxic, flammable liquid consisting of a complex mixture of hydrocarbons Petroleum engineering petroleum geology pdf petroleum pipelines company petrochemicals petroleum science and technology petroleum geology ppt petroleum products production engineering petroleum products ppt petroleum pollution petroleum economics petroleum economist refining refining processes refining economics
visit us if you want to make mony from your home
if you want to make free blog and you have the idea but not the experience we can prove it to you to earn money from blogging
How to keep your child's health in winter
Winter
is the time may have a negative impact on the immune system of the
child, bearing in mind that the system of child food in winter should
contain vegetables, fruits and meat soft and herbs because all those
elements will help in maintaining the health of the child. The
mother should know that her system if food has been lacking for some of
the elements, the child will not be able to get a share of the
vitamins, minerals and antioxidants which will lead to his immune
system. Encouraging
your child to eat foods that contain nutrients piety of the immune
system is will help the child to resist colds and various winter
diseases. Certainly,
your child will get sick in the winter, but the mother through a
healthy diet and through a range of tips and various steps to maintain
her strong and healthy in the winter.
The regular hand washing a simple and effective way to protect children from colds and winter illnesses. We must help your child to wash his hands with soap and warm water after entering the bathroom and before eating and after returning from school or from a friend's house with the situation in mind that you are also must take care wash your hands before preparing food and before changing a baby's diaper and even before clearing his nose. And you should also mother to make sure whether your child is washing his hands well or not is in custody.
Strive to be your child's clothes in a warm winter to ensure its survival and must include the child's wardrobe in winter jacket and gloves and a hat and some other elements that will work on the protection of children from different weather elements in the winter. The child if he scans his eye or nose with his hands, it would be that way transmit germs that quickly seep to the bloodstream has your child should learn how to use the tissue and also be used when you cough or sneeze.
Child usually recovers quickly after a cold or infection in the high temperature, but his immune system may be somewhat strained, making it susceptible to multiple بنزلات cold. Do you can use garlic, which contains the element strengthens the immune allicin, bearing in mind that you can write by adding garlic to your pasta or rice dishes or different fish dishes. Rather than progressive for your baby foods are canned in advance and contain a high proportion of additions and high in sugar, salt and fat and my feet for your child in return vegetables and fruits with the situation in mind that even if resisted your child this you should be doing to enter healthy foods in a gradual manner through breakfasts lunch and dinner and snacks.
Basil is one of the powerful herbs that will protect the child from diseases winter and you can write enter your basil for meals which're prepared for your child, such as tomato sauce, pizza and different kinds of soup. Do not give your child drinks, frozen in winter because this may reduce the ability of the immune system to resist colds and various winter diseases. Not given a bath for your child and Valuation wash his hair before he goes to sleep immediately because this would undermine immunity and therefore try to be baby bath sufficiently in advance of the date and dinner to dry his hair.
The child goes to bed at a reasonable date is very important because the child would not be able to recover in general if he does not get the number from nine to ten hours of sleep. Avoid giving your child a lot of candy or make watching television in the evening.Winter is the time may have a negative impact on the immune system of the child, bearing in mind that the system of child food in winter should contain vegetables, fruits and meat soft and herbs because all those elements will help in maintaining the health of the child. The mother should know that her system if food has been lacking for some of the elements, the child will not be able to get a share of the vitamins, minerals and antioxidants which will lead to his immune system. Encouraging your child to eat foods that contain nutrients piety of the immune system is will help the child to resist colds and various winter diseases. Certainly, your child will get sick in the winter, but the mother through a healthy diet and through a range of tips and various steps to maintain her strong and healthy in the winter.
The regular hand washing a simple and effective way to protect children from colds and winter illnesses. We must help your child to wash his hands with soap and warm water after entering the bathroom and before eating and after returning from school or from a friend's house with the situation in mind that you are also must take care wash your hands before preparing food and before changing a baby's diaper and even before clearing his nose. And you should also mother to make sure whether your child is washing his hands well or not is in custody.
Strive to be your child's clothes in a warm winter to ensure its survival and must include the child's wardrobe in winter jacket and gloves and a hat and some other elements that will work on the protection of children from different weather elements in the winter. The child if he scans his eye or nose with his hands, it would be that way transmit germs that quickly seep to the bloodstream has your child should learn how to use the tissue and also be used when you cough or sneeze.
Child usually recovers quickly after a cold or infection in the high temperature, but his immune system may be somewhat strained, making it susceptible to multiple بنزلات cold. Do you can use garlic, which contains the element strengthens the immune allicin, bearing in mind that you can write by adding garlic to your pasta or rice dishes or different fish dishes. Rather than progressive for your baby foods are canned in advance and contain a high proportion of additions and high in sugar, salt and fat and my feet for your child in return vegetables and fruits with the situation in mind that even if resisted your child this you should be doing to enter healthy foods in a gradual manner through breakfasts lunch and dinner and snacks.
Basil is one of the powerful herbs that will protect the child from diseases winter and you can write enter your basil for meals which're prepared for your child, such as tomato sauce, pizza and different kinds of soup. Do not give your child drinks, frozen in winter because this may reduce the ability of the immune system to resist colds and various winter diseases. Not given a bath for your child and Valuation wash his hair before he goes to sleep immediately because this would undermine immunity and therefore try to be baby bath sufficiently in advance of the date and dinner to dry his hair.
The child goes to bed at a reasonable date is very important because the child would not be able to recover in general if he does not get the number from nine to ten hours of sleep. Avoid giving your child a lot of candy or make watching television in the evening.
The regular hand washing a simple and effective way to protect children from colds and winter illnesses. We must help your child to wash his hands with soap and warm water after entering the bathroom and before eating and after returning from school or from a friend's house with the situation in mind that you are also must take care wash your hands before preparing food and before changing a baby's diaper and even before clearing his nose. And you should also mother to make sure whether your child is washing his hands well or not is in custody.
Strive to be your child's clothes in a warm winter to ensure its survival and must include the child's wardrobe in winter jacket and gloves and a hat and some other elements that will work on the protection of children from different weather elements in the winter. The child if he scans his eye or nose with his hands, it would be that way transmit germs that quickly seep to the bloodstream has your child should learn how to use the tissue and also be used when you cough or sneeze.
Child usually recovers quickly after a cold or infection in the high temperature, but his immune system may be somewhat strained, making it susceptible to multiple بنزلات cold. Do you can use garlic, which contains the element strengthens the immune allicin, bearing in mind that you can write by adding garlic to your pasta or rice dishes or different fish dishes. Rather than progressive for your baby foods are canned in advance and contain a high proportion of additions and high in sugar, salt and fat and my feet for your child in return vegetables and fruits with the situation in mind that even if resisted your child this you should be doing to enter healthy foods in a gradual manner through breakfasts lunch and dinner and snacks.
Basil is one of the powerful herbs that will protect the child from diseases winter and you can write enter your basil for meals which're prepared for your child, such as tomato sauce, pizza and different kinds of soup. Do not give your child drinks, frozen in winter because this may reduce the ability of the immune system to resist colds and various winter diseases. Not given a bath for your child and Valuation wash his hair before he goes to sleep immediately because this would undermine immunity and therefore try to be baby bath sufficiently in advance of the date and dinner to dry his hair.
The child goes to bed at a reasonable date is very important because the child would not be able to recover in general if he does not get the number from nine to ten hours of sleep. Avoid giving your child a lot of candy or make watching television in the evening.Winter is the time may have a negative impact on the immune system of the child, bearing in mind that the system of child food in winter should contain vegetables, fruits and meat soft and herbs because all those elements will help in maintaining the health of the child. The mother should know that her system if food has been lacking for some of the elements, the child will not be able to get a share of the vitamins, minerals and antioxidants which will lead to his immune system. Encouraging your child to eat foods that contain nutrients piety of the immune system is will help the child to resist colds and various winter diseases. Certainly, your child will get sick in the winter, but the mother through a healthy diet and through a range of tips and various steps to maintain her strong and healthy in the winter.
The regular hand washing a simple and effective way to protect children from colds and winter illnesses. We must help your child to wash his hands with soap and warm water after entering the bathroom and before eating and after returning from school or from a friend's house with the situation in mind that you are also must take care wash your hands before preparing food and before changing a baby's diaper and even before clearing his nose. And you should also mother to make sure whether your child is washing his hands well or not is in custody.
Strive to be your child's clothes in a warm winter to ensure its survival and must include the child's wardrobe in winter jacket and gloves and a hat and some other elements that will work on the protection of children from different weather elements in the winter. The child if he scans his eye or nose with his hands, it would be that way transmit germs that quickly seep to the bloodstream has your child should learn how to use the tissue and also be used when you cough or sneeze.
Child usually recovers quickly after a cold or infection in the high temperature, but his immune system may be somewhat strained, making it susceptible to multiple بنزلات cold. Do you can use garlic, which contains the element strengthens the immune allicin, bearing in mind that you can write by adding garlic to your pasta or rice dishes or different fish dishes. Rather than progressive for your baby foods are canned in advance and contain a high proportion of additions and high in sugar, salt and fat and my feet for your child in return vegetables and fruits with the situation in mind that even if resisted your child this you should be doing to enter healthy foods in a gradual manner through breakfasts lunch and dinner and snacks.
Basil is one of the powerful herbs that will protect the child from diseases winter and you can write enter your basil for meals which're prepared for your child, such as tomato sauce, pizza and different kinds of soup. Do not give your child drinks, frozen in winter because this may reduce the ability of the immune system to resist colds and various winter diseases. Not given a bath for your child and Valuation wash his hair before he goes to sleep immediately because this would undermine immunity and therefore try to be baby bath sufficiently in advance of the date and dinner to dry his hair.
The child goes to bed at a reasonable date is very important because the child would not be able to recover in general if he does not get the number from nine to ten hours of sleep. Avoid giving your child a lot of candy or make watching television in the evening.
What is the reason that some thing for taking medication before eating specifically and not after
Why taken some medication before eating?
Because the food may reduce the absorption of some drugs
And thus reduces the desired therapeutic effect
Or food may cause cracking some drugs
And thus lost its impact.
So take these medicines hour before eating or two hours after eating.
Because the food may reduce the absorption of some drugs
And thus reduces the desired therapeutic effect
Or food may cause cracking some drugs
And thus lost its impact.
So take these medicines hour before eating or two hours after eating.
Do you know the time required to get rid of calories from drinking one pack of Pepsi or Coca-Cola?
Sure, drink Pepsi or Coke Cola is nice to have a sometimes
Take five minutes of time to change my. But we need a full hour to get rid
Of calories from drinking that per box. Where researchers stressed
In one of the universities in the United States on the need to raise awareness
Between people and as especially young people and children who are the largest segment consumers
And to reduce the drinking of these materials in abundance, as well as to educate the users
Ban do some exercise after eating to get rid of
Of excess calories. As reported by the Middle East News Agency
Take five minutes of time to change my. But we need a full hour to get rid
Of calories from drinking that per box. Where researchers stressed
In one of the universities in the United States on the need to raise awareness
Between people and as especially young people and children who are the largest segment consumers
And to reduce the drinking of these materials in abundance, as well as to educate the users
Ban do some exercise after eating to get rid of
Of excess calories. As reported by the Middle East News Agency
Effective ways to control the food and lose weight easily
Learning
to identify quantities we eat: through the use of tools to help you
measure the amount of food and size fluid custom Kkos for the measure,
teaspoon or food, or the balance of the special weight of foods.You
can also use some of the criteria theory, which enables us to estimate
the size of the food once seen, so comparing it with things we know its
size in advance, as an example, the share of cooked meat or fish or
chicken-size tray Securities king (note that you are allowed is from 2 -
3 share a day).There
are also more easy metrics to the naked eye: for example, a half cup is
ball-sized ice cream, one cup is the size of a tennis ball, 25 grams of
cheese is the size of domino Akecat.Use
dishes quotas food: Log acquisition dishes and cups (or Mjat) smaller
instead of dishes, cups large amounts in size, which is usually what we
use, and measure the amount contained in a one-time only so you know how
much food and drink you eat.Do
not put the main dish on the dining table: Avoid placing the main dish
on the dining table, and distribution must dishes in advance, it will
help you in reducing your appetite to eat the dish last as long as it is
far away from your eyes, and far from your fingertips.Add milk first before adding the hot water for beverages such as tea, Alnskafah or drink hot chocolate:Add
skim milk to the cup or jewelry before adding tea or Alnskafah can
determine the amount of milk consumed, and thus amount of calories you
eat each drink beverages.Mouth
measuring the amount of oil used in the preparation of food: It is
important to do so Valziot even species health including as olive oil
and sunflower oil containing calories by high, so do not put oil in the
pan directly or on food before measuring and quantify, so we know much
you eat them (Note: g oil gives 9 calories, while gram carbohydrates or proteins be given 4 calories).Control
the amount of food you eat at the restaurant: by addressing half the
meal, or with friends in a meal, but you're dealing with authority ask
to be placed Sous your power on the side of the dish and not to add to
the power, and use the types of sauces and light a few calories, but
enough by spoon teaspoon of olive oil with a little vinegar or mustard.Add
vegetables to your diet: try eating a plate of vegetable soup at the
beginning of the food, this dish is characterized as low in content of
calories, eating this dish before eating any meal will make you feel
fuller quickly. Also
add vegetables to a dish or sandwich you eat in order to increase the
size of the meal, and up to the satiety quickly without having to eat
more calories.Hearsnobody
phantom hints hunger: eat when you are already hungry, and stop eating
once a feeling of satiety or fullness, and do not forget to drink water
regularly, because sometimes many thirst cause the feeling of hunger.Drink
large amounts of water: water makes you feel full stomach, thirst may
also be a reason to feel hungry as well as we have. Chew food well:The chew and longer given brain enough time to register a feeling of fullness more quickly, and keeps you from eating more food.
Diet to reach the ideal weight
Reader sent us say: I'm a lady in the thirty-second of age and has two children, and since started to follow the different diets to lose my weight with my practice of walking on an almost daily basis, and lost about twenty kilometers this year.
And now arrived and adultery to 67 kilograms, and I would like to reach an ideal weight, knowing that the longitudinal 160 cm, and I want to get to 60 kilograms, what is the diet which can make me lose this excess weight?
Answer this question, Dr. Khaled Youssef Specialist obesity and thinness, a member of the Egyptian Society for the Study of Obesity, a member of the American Society of obesity, saying: weight-loss residual and access to ideal weight are advised Ms. follow the diet and consists of the following:
Breakfast 5 Tamrat + cup skim milk. Hour later: - cup pineapple juice without sugar.
Food
Can of tuna from the oil refinery
Or 1/4 Farkha + salad + loaf of age. Hour later: - fruit orange or apple or pear.
Dinner
Toast + piece Lite cheese with basil and thyme + green salad. While adhering to some important tips: drink 3 cups of water before eating. Hiking on a daily basis for at least half an hour.
And now arrived and adultery to 67 kilograms, and I would like to reach an ideal weight, knowing that the longitudinal 160 cm, and I want to get to 60 kilograms, what is the diet which can make me lose this excess weight?
Answer this question, Dr. Khaled Youssef Specialist obesity and thinness, a member of the Egyptian Society for the Study of Obesity, a member of the American Society of obesity, saying: weight-loss residual and access to ideal weight are advised Ms. follow the diet and consists of the following:
Breakfast 5 Tamrat + cup skim milk. Hour later: - cup pineapple juice without sugar.
Food
Can of tuna from the oil refinery
Or 1/4 Farkha + salad + loaf of age. Hour later: - fruit orange or apple or pear.
Dinner
Toast + piece Lite cheese with basil and thyme + green salad. While adhering to some important tips: drink 3 cups of water before eating. Hiking on a daily basis for at least half an hour.
Eliminate fat foods final
There
are foods that can help us to stimulate the metabolic system and thus
burn calories and fat, but will, of course, if carried out through
effective diet and Weight Loss Program.1. Apple:Do you remember the old adage: "An apple a day keeps the doctor away," Well, is not only a doctor, but excessive fines as well. Apple contains a higher percentage of pectin, a soluble fiber.
2: Garlic:Garlic one of the most effective foods to burn fat. Garlic contains allicin compound, which has antibacterial effects and helps reduce cholesterol and unhealthy fats.
3. Tomatoes:Tomatoes are very effective and worthy additions to your diet. They are wonderful in your fight against excess weight as well as also important to prevent and repel cancer and high blood pressure.
4: the islands:Add carrots to a wonderful diet and is very effective in combating obesity and fat burning. Why? Because islands fill the stomach and thus will not stay for dessert. If you are able to exploit this trick at every meal, it will help you to get rid of excess weight in a week.
5: orange:Oranges rich in vitamin C also has fat-burning properties. Coupled with exercise orange is an effective way to lose fat, and here we are talking about and not juice oranges.
6: Mango:Mango packed in fiber and low in calories, and the taste is delicious and rich and confectionery Stgnek.
7: spinach:Popeye gets his strength from spinach and you too. Valsbank very healthy food. Spinach contains lots of iron; an exceptional food helps to prevent cancer
2: Garlic:Garlic one of the most effective foods to burn fat. Garlic contains allicin compound, which has antibacterial effects and helps reduce cholesterol and unhealthy fats.
3. Tomatoes:Tomatoes are very effective and worthy additions to your diet. They are wonderful in your fight against excess weight as well as also important to prevent and repel cancer and high blood pressure.
4: the islands:Add carrots to a wonderful diet and is very effective in combating obesity and fat burning. Why? Because islands fill the stomach and thus will not stay for dessert. If you are able to exploit this trick at every meal, it will help you to get rid of excess weight in a week.
5: orange:Oranges rich in vitamin C also has fat-burning properties. Coupled with exercise orange is an effective way to lose fat, and here we are talking about and not juice oranges.
6: Mango:Mango packed in fiber and low in calories, and the taste is delicious and rich and confectionery Stgnek.
7: spinach:Popeye gets his strength from spinach and you too. Valsbank very healthy food. Spinach contains lots of iron; an exceptional food helps to prevent cancer
Tips for dry skins owners
Although dry skin may accompany some throughout the year, but it is usually more severe in winter.The following tips to help overcome this drought, which sometimes socializing with itching:- Avoid bathing and washing hands with very hot water, but prefers to use lukewarm water.
The experts also advised not to continue bathing for more than 5-10 minutes.- Avoid soap that contains alcohol or fragrance, but the use of Algsolat creamy moisturizing shower and wash your hands.
Preferably This Algsolat contains material Alseramed ceramides,It is a synthetic working to help the skin retain its moisture. It is noteworthy that the substance Alseramed natural skin already exist, but they gradually lose with age.- After bathing, national draining your body Balrepett gently using a soft towel, not quickly Pferkh.- Nationalist using moisturizing cream immediately after drying your body.- Nationalist using skin moisturizers that contain petroleum jelly petroleum jelly. Or glycerin. These soft materials help to regenerate the skin barrier which prevents the loss of moisture.- Use a moisturizer cream to your hands and then nationalist wear gloves before going out in cold weather, especially if the skin very dry hands.- Before going to sleep, use a thick moisturizer cream for your feet and nationalist wear cotton socks.- As the heat of the sun is a reason for dry skin and Khcontha, even in winter, it is recommended using creams containing sunscreen to be preventable factor 30, and so on throughout the year.It is worth noting that some studies have found that the use of oral omega-3 fatty acid helps to soften the skin severe roughness in a short time. He stated Dr. Andrew Weill, a skin health expert, that after the use of these pills for six weeks only, appeared a clear improvement on the skin, hair and nails users.
The experts also advised not to continue bathing for more than 5-10 minutes.- Avoid soap that contains alcohol or fragrance, but the use of Algsolat creamy moisturizing shower and wash your hands.
Preferably This Algsolat contains material Alseramed ceramides,It is a synthetic working to help the skin retain its moisture. It is noteworthy that the substance Alseramed natural skin already exist, but they gradually lose with age.- After bathing, national draining your body Balrepett gently using a soft towel, not quickly Pferkh.- Nationalist using moisturizing cream immediately after drying your body.- Nationalist using skin moisturizers that contain petroleum jelly petroleum jelly. Or glycerin. These soft materials help to regenerate the skin barrier which prevents the loss of moisture.- Use a moisturizer cream to your hands and then nationalist wear gloves before going out in cold weather, especially if the skin very dry hands.- Before going to sleep, use a thick moisturizer cream for your feet and nationalist wear cotton socks.- As the heat of the sun is a reason for dry skin and Khcontha, even in winter, it is recommended using creams containing sunscreen to be preventable factor 30, and so on throughout the year.It is worth noting that some studies have found that the use of oral omega-3 fatty acid helps to soften the skin severe roughness in a short time. He stated Dr. Andrew Weill, a skin health expert, that after the use of these pills for six weeks only, appeared a clear improvement on the skin, hair and nails users.
Beware cover your head while sleeping
Beware cover your head while sleeping there is usually a serious do it a lot of people do not know what damage this habit wrong which cover the head during sleep, the head covered during sleep completely (whether cover mattress or padding) bad habit and dangerous because it increases the concentration of carbon dioxide and a fewthe proportion of oxygen in the space to breathe and this will be like smoking and lead to disaster involving brain cells and may lead to damage to brain cells and Dmorha and stop their growth .... And thus the loss of the brain the ability to carry out its functions effectively and efficiently.
Expel the dead from their graves get for not paying fees graves already happening in Guatemala
Probably will not believe this but it is already happening in Guatemala, where poverty prevails there and families of the dead could not pay the use fee graves. Mummified corpse was exhumed from the grave in Guatemala and assigned on the wall in preparation for the arrival of the family of the deceased to be recovered. Where municipal employees in the capital removing the dead from their graves for the non-payment of their parents to the grave charges and develop metadata for each corpse, and then placed in a mass grave waiting for the people
The dead to be recovered.
The dead to be recovered.
Show: 10 Best Foods, useful for the kidneys
Kidneys
are small members of the body does not exceed, each one the size of the
wrist, but played a great role, function of the kidneys is to regulate
the chemicals and fluids in the body, cleaning waste from the blood, and
given the importance of the role played by the kidneys .. We choose foods that food Tmayorma support, and avoid exposure to damage. Here's
a list of key 10 foods benefit kidney: 1 - red pepper red pepper few in
«potassium», but the taste is rich, characterized as containing vitamin
«a» «c» and «B 6» and acid «folic» Many of the fiber, containing red
pepper also on Article «lycopene» antioxidant, which protects against cancer. 2
- cabbage cabbage goes «Alvaetukemejkalz» which fights cancer and
improve the health of the heart and blood, as well as cabbage is rich in
vitamin 'c' and 'b 6' acid 'folate and fiber, as it is low in' K '. 3
- cauliflower, broccoli is rich in vitamin 'c' and fiber Alfolaat,
which also contains compounds help to break up kidney toxic substances
that may harm the membranes. 4
- garlic, onions, garlic helps prevent infections and reduce
cholesterol, which is rich in antioxidants, which makes it an ideal
addition to many dishes. The
onions, is a rich, textured chemicals prevent the deposition of fat in
the blood vessels, inhibit oxidation, onions also contain a little
'potassium', but rich «chromium, which helps to digest fats, proteins
and Alcarbohedrics. 5 - apple apple fiber rich .. And
features to reduce cholesterol and prevent constipation and prevention
of cancer and heart disease, as the saying goes, England .. «An apple a day ... Taqi of the visit to the doctor, you can eat it or eat juice. 6
- berries, strawberry, cherry, raspberry and strawberries are rich in
vitamin 'c' and fiber and «Almnjanez», are friends of the kidneys, and
their antioxidants help to build a healthy body. The cherry .. It is characterized in antioxidants, as well as «Alvaetukemejkalz», which protect the heart and prevent infections. 7
- red grapes red grapes prevents oxidation and blood clots and helps to
ease the flow of blood, make sure to pick the grapes with the red skin
or dark purple because the richest materials that prevent oxidation. 8
- egg whites, egg whites of the richest foods in protein, and contains
'phosphorus' much less than the egg yolk or meat, so it is better for
the kidneys. 9
- rich fish 'omega-3' of the many advantages it contains
anti-inflammatory substances, and which protect against cancer, heart
disease and blood vessels, Stagdi oils 'omega-3' in tuna, salmon and
herring. 10
- Olive oil Olive oil contains anti-inflammatory substances and
antioxidant, so researchers have found that cooking with olive oil
healthier than others, so you need olive oil
Lectures on Heat (3)
Thermal Expansion and the Gas Law
Coefficients of Expansion
Almost all materials expand on heating—the most famous exception being water, which contracts as it is warmed from 0 degrees Celsius to 4 degrees. This is actually a good thing, because as freezing weather sets in, the coldest water, which is about to freeze, is less dense than slightly warmer water, so rises to the top of a lake and the ice begins to form there. For almost all other liquids, solidification on cooling begins at the bottom of the container. So, since water behaves in this weird way, ice skating is possible! Also, as a matter of fact, life in lakes is possible—the ice layer that forms insulates the rest of the lake water from very cold air, so fish can make it through the winter.
Linear Expansion
The coefficient of linear expansion α of a given material, for example a bar of copper, at a given temperature is defined as the fractional increase in length that takes place on heating through one degree:
Almost all materials expand on heating—the most famous exception being water, which contracts as it is warmed from 0 degrees Celsius to 4 degrees. This is actually a good thing, because as freezing weather sets in, the coldest water, which is about to freeze, is less dense than slightly warmer water, so rises to the top of a lake and the ice begins to form there. For almost all other liquids, solidification on cooling begins at the bottom of the container. So, since water behaves in this weird way, ice skating is possible! Also, as a matter of fact, life in lakes is possible—the ice layer that forms insulates the rest of the lake water from very cold air, so fish can make it through the winter.
Linear Expansion
The coefficient of linear expansion α of a given material, for example a bar of copper, at a given temperature is defined as the fractional increase in length that takes place on heating through one degree:
Of course, α might vary with temperature (it does for water, as we just mentioned) but in fact for most materials it stays close to constant over wide temperature ranges.
Volume Expansion
For liquids and gases, the natural measure of expansion is the coefficient of volume expansion,β.
For liquids and gases, the natural measure of expansion is the coefficient of volume expansion,β.
Of course, on heating a bar of copper, clearly the volume as well as the length increases—the bar expands by an equal fraction in all directions (this could be experimentally verified, or you could just imagine a cube of copper, in which case all directions look the same).
The volume of a cube of copper of side L is V = L3. Suppose we heat it through one degree. Putting together the definitions of ,αβabove,
The volume of a cube of copper of side L is V = L3. Suppose we heat it through one degree. Putting together the definitions of ,αβabove,
Gas Pressure Increase with Temperature
In 1702, Amontons discovered a linear increase of P with T for air, and found P to increase about 33% from the freezing point of water to the boiling point of water.
That is to say, he discovered that if a container of air were to be sealed at 0°C, at ordinary atmospheric pressure of 15 pounds per square inch, and then heated to 100°C but kept at the same volume, the air would now exert a pressure of about 20 pounds per square inch on the sides of the container. (Of course, strictly speaking, the container will also have increased in size, that would lower the effect—but it’s a tiny correction, about ½% for copper, even less for steel and glass.)
Remarkably, Amontons discovered, if the gas were initially at a pressure of thirty pounds per square inch at 0°C, on heating to 100°C the pressure would go to about 40 pounds per square inch—so the percentage increase in pressure was the same for any initial pressure: on heating through 100°C, the pressure would always increase by about 33%.
Furthermore, the result turned out to be the same for different gases!
That is to say, he discovered that if a container of air were to be sealed at 0°C, at ordinary atmospheric pressure of 15 pounds per square inch, and then heated to 100°C but kept at the same volume, the air would now exert a pressure of about 20 pounds per square inch on the sides of the container. (Of course, strictly speaking, the container will also have increased in size, that would lower the effect—but it’s a tiny correction, about ½% for copper, even less for steel and glass.)
Remarkably, Amontons discovered, if the gas were initially at a pressure of thirty pounds per square inch at 0°C, on heating to 100°C the pressure would go to about 40 pounds per square inch—so the percentage increase in pressure was the same for any initial pressure: on heating through 100°C, the pressure would always increase by about 33%.
Furthermore, the result turned out to be the same for different gases!
Finding a Natural Temperature Scale
In class, we plotted air pressure as a function of temperature for a fixed volume of air, by making several measurements as the air was slowly heated (to give it a chance to all be at the same temperature at each stage). We found a straight line. On the graph, we extended the line backwards, to see how the pressure would presumably drop on cooling the air. We found the remarkable prediction that the pressure should drop to zero at a temperature of about −273°C.
In fact, if we’d done the cooling experiment, we would have found that air doesn’t actually follow the line all the way down, but condenses to a liquid at around −200°C. However, helium gas stays a gas almost to −270°C, and follows the line closely.
In class, we plotted air pressure as a function of temperature for a fixed volume of air, by making several measurements as the air was slowly heated (to give it a chance to all be at the same temperature at each stage). We found a straight line. On the graph, we extended the line backwards, to see how the pressure would presumably drop on cooling the air. We found the remarkable prediction that the pressure should drop to zero at a temperature of about −273°C.
In fact, if we’d done the cooling experiment, we would have found that air doesn’t actually follow the line all the way down, but condenses to a liquid at around −200°C. However, helium gas stays a gas almost to −270°C, and follows the line closely.
We shall discuss the physics of gases, and the interpretation of this, much more fully in a couple of lectures. For now, the important point is that this suggests a much more natural temperature scale than the Celsius one: we should take −273°C as the zero of temperature! For one thing, if we do that, the pressure/temperature relationship for a gas becomes beautifully simple:
P ∝ T
This temperature scale, in which the degrees have the same size as in Celsius, is called the Kelvin or absolute scale. Temperatures are written 300K. To get from Celsius to Kelvin, just add 273 (strictly speaking, 273.15).
An Ideal Gas
Physicists at this point introduce the concept of an “Ideal Gas”. This is like the idea of a frictionless surface: it doesn’t exist in nature, but it is a very handy approximation to some real systems, and makes problems much easier to handle mathematically. The ideal gas is one for which for all temperatures, so helium is close to ideal over a very wide range, and air is close to ideal at ordinary atmospheric temperatures and above.
P ∝ T
This temperature scale, in which the degrees have the same size as in Celsius, is called the Kelvin or absolute scale. Temperatures are written 300K. To get from Celsius to Kelvin, just add 273 (strictly speaking, 273.15).
An Ideal Gas
Physicists at this point introduce the concept of an “Ideal Gas”. This is like the idea of a frictionless surface: it doesn’t exist in nature, but it is a very handy approximation to some real systems, and makes problems much easier to handle mathematically. The ideal gas is one for which for all temperatures, so helium is close to ideal over a very wide range, and air is close to ideal at ordinary atmospheric temperatures and above.
Lectures on Heat (2)
Thermal Equilibrium and the Zeroth Law of Thermodynamics
Once the thermometer came to be widely used, more precise observations of temperature and (as we shall see) heat flow became possible. Joseph Black, a professor at the University of Edinburgh in the 1700’s, noticed that a collection of objects at different temperatures, if brought together, will all eventually reach the same temperature.
As he wrote, “By the use of these instruments [thermometers] we have learned, that if we take 1000, or more, different kinds of matter, such as metals, stones, salts, woods, cork, feathers, wool, water and a variety of other fluids, although they be all at first of different heats, let them be placed together in a room without a fire, and into which the sun does not shine, the heat will be communicated from the hotter of these bodies to the colder, during some hours, perhaps, or the course of a day, at the end of which time, if we apply a thermometer to all of them in succession, it will point to precisely the same degree.”
We say nowadays that bodies in “thermal contact” eventually come into “thermal equilibrium”—which means they finally attain the same temperature, after which no further heat flow takes place. This is equivalent to:
The Zeroth Law of Thermodynamics:
If two objects are in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
The “third body” in a practical situation is just the thermometer.
It’s perhaps worth pointing out that this trivial sounding statement certainly wasn’t obvious before the invention of the thermometer. With only the sense of touch to go on, few people would agree that a piece of wool and a bar of metal, both at 0°C, were at the same temperature.
Measuring Heat Flow: a Unit of Heat
The next obvious question is, can we get more quantitative about this “flow of heat” that takes place between bodies as they move towards thermal equilibrium? For example, suppose I reproduce one of Fahrenheit’s experiments, by taking 100 ccs of water at 100°F, and 100ccs at 150°F, and mix them together in an insulated jug so little heat escapes. What is the final temperature of the mix?
Of course, it’s close to 125°F—not surprising, but it does tell us something! It tells us that the amount of heat required to raise the temperature of 100 cc of water from 100°F to 125°F is exactly the same as the amount needed to raise it from 125°F to 150°F. A series of such experiments (done by Fahrenheit, Black and others) established that it always took the same amount of heat to raise the temperature of 1 cc of water by one degree.
This makes it possible to define a unit of heat. Perhaps unfairly to Fahrenheit,
1 calorie is the heat required to raise the temperature of 1 gram of water by 1 degree Celsius.
(Celsius also lived in the early 1700’s. His scale has the freezing point of water as 0°C, the boiling point as 100°C. Fahrenheit’s scale is no longer used in science, but lives on in engineering in the US, and in the British Thermal Unit, which is the heat required to raise the temperature of one pound of water by 1°F.)
Specific Heats and Calorimetry
First, let’s define specific heat:
The specific heat of a substance is the heat required in calories to raise the temperature of 1 gram by 1 degree Celsius.
As Fahrenheit continues his measurements of heat flow, it quickly became evident that for different materials, the amount of heat needed to raise the temperature of one gram by one degree could be quite different. For example, it had been widely thought before the measurements were made, that one cc of Mercury, being a lot heavier than one cc of water, would take more heat to raise its temperature by one degree. This proved not to be the case—Fahrenheit himself made the measurement. In an insulating container, called a “calorimeter” he added 100ccs of water at 100°F to 100ccs of mercury at 150°F, and stirred so they quickly reached thermal equilibrium.
Question: what do you think the final temperature was? Approximately?
Answer: The final temperature was, surprisingly, about 120°F. 100 cc of water evidently “contained more heat” than 100 cc of mercury, despite the large difference in weight!
This technique, called calorimetry, was widely used to find the specific heats of many different substances, and at first no clear pattern emerged. It was puzzling that the specific heat of mercury was so low compared with water! As more experiments on different substances were done, it gradually became evident that heavier substances, paradoxically, had lower specific heats.
A Connection With Atomic Theory
Meanwhile, this quantitative approach to scientific observation had spread to chemistry. Towards the end of the 1700’s, Lavoisier weighed chemicals involved in reactions before and after the reaction. This involved weighing the gases involved, so had to be carried out in closed containers, so that, for example, the weight of oxygen used and the carbon dioxide, etc., produced would accounted for in studying combustion. The big discovery was that mass was neither created nor destroyed. This had not been realized before because no one had weighed the gases involved. It made the atomic theory suddenly more plausible, with the idea that maybe chemical reactions were just rearrangements of atoms into different combinations.
Lavoisier also clarified the concept of an element, an idea that was taken up in about 1800 by John Dalton, who argues that a given compound consisted of identical molecules, made up of elementary atoms in the same proportion, such as H2O (although that was thought initially to be HO). This explained why, when substances reacted chemically, such as the burning of hydrogen to form water, it took exactly eight grams of oxygen for each gram of hydrogen. (Well, you could also produce H2O2 under the right conditions, with exactly sixteen grams of oxygen to one of hydrogen, but the simple ratios of amounts of oxygen needed for the two reactions were simply explained by different molecular structures, and made the atomic hypothesis even more plausible.)
Much effort was expended carefully weighing the constituents in many chemical reactions, and constructing diagrams of the molecules. The important result of all this work was that it became possible to list the relative weights of the atoms involved. For example, the data on H2O and H2O2 led to the conclusion that an oxygen atom weighed sixteen times the weight of a hydrogen atom.
It must be emphasized, though, that these results gave no clue as to the actual weights of atoms! All that was known was that atoms were too small to see in the best microscopes. Nevertheless, knowing the relative weights of some atoms in 1820 led to an important discovery. Two professors in France, Dulong and Petit, found that for a whole series of elements the product of atomic weight and specific heat was the same!
The significance of this, as they pointed out, was that the “specific heat”, or heat capacity, of each atom was the same—a piece of lead and a piece of zinc having the same number of atoms would have the same heat capacity. So heavier atoms absorbed no more heat than lighter atoms for a given rise in temperature. This partially explained why mercury had such a surprisingly low heat capacity. Of course, having no idea how big the atoms might be, they could go no further. And, indeed, many of their colleagues didn’t believe in atoms anyway, so it was hard to convince them of the significance of this discovery.
Latent Heat
One of Black’s experiments was to set a pan of water on a steady fire and observe the temperature
as a function of time. He found it steadily increased, reflecting the supply of heat from the fire, until the water began to boil, whereupon the temperature stayed the same for a long time. The steam coming off was at the same (boiling) temperature as the water. So what was happening to the heat being supplied? Black correctly concluded that heat needed to be supplied to change water from its liquid state to its gaseous state, that is, to steam. In fact, a lot of heat had to be supplied: 540 calories per gram, as opposed to the mere 100 calories per gram needed to bring it from the freezing temperature to boiling. He also discovered that it took 80 calories per gram to melt ice into water, with no rise in temperature. This heat is released when the water freezes back to ice, so it is somehow “hidden” in the water. He called it latent heat, meaning hidden heat.
Once the thermometer came to be widely used, more precise observations of temperature and (as we shall see) heat flow became possible. Joseph Black, a professor at the University of Edinburgh in the 1700’s, noticed that a collection of objects at different temperatures, if brought together, will all eventually reach the same temperature.
As he wrote, “By the use of these instruments [thermometers] we have learned, that if we take 1000, or more, different kinds of matter, such as metals, stones, salts, woods, cork, feathers, wool, water and a variety of other fluids, although they be all at first of different heats, let them be placed together in a room without a fire, and into which the sun does not shine, the heat will be communicated from the hotter of these bodies to the colder, during some hours, perhaps, or the course of a day, at the end of which time, if we apply a thermometer to all of them in succession, it will point to precisely the same degree.”
We say nowadays that bodies in “thermal contact” eventually come into “thermal equilibrium”—which means they finally attain the same temperature, after which no further heat flow takes place. This is equivalent to:
The Zeroth Law of Thermodynamics:
If two objects are in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
The “third body” in a practical situation is just the thermometer.
It’s perhaps worth pointing out that this trivial sounding statement certainly wasn’t obvious before the invention of the thermometer. With only the sense of touch to go on, few people would agree that a piece of wool and a bar of metal, both at 0°C, were at the same temperature.
Measuring Heat Flow: a Unit of Heat
The next obvious question is, can we get more quantitative about this “flow of heat” that takes place between bodies as they move towards thermal equilibrium? For example, suppose I reproduce one of Fahrenheit’s experiments, by taking 100 ccs of water at 100°F, and 100ccs at 150°F, and mix them together in an insulated jug so little heat escapes. What is the final temperature of the mix?
Of course, it’s close to 125°F—not surprising, but it does tell us something! It tells us that the amount of heat required to raise the temperature of 100 cc of water from 100°F to 125°F is exactly the same as the amount needed to raise it from 125°F to 150°F. A series of such experiments (done by Fahrenheit, Black and others) established that it always took the same amount of heat to raise the temperature of 1 cc of water by one degree.
This makes it possible to define a unit of heat. Perhaps unfairly to Fahrenheit,
1 calorie is the heat required to raise the temperature of 1 gram of water by 1 degree Celsius.
(Celsius also lived in the early 1700’s. His scale has the freezing point of water as 0°C, the boiling point as 100°C. Fahrenheit’s scale is no longer used in science, but lives on in engineering in the US, and in the British Thermal Unit, which is the heat required to raise the temperature of one pound of water by 1°F.)
Specific Heats and Calorimetry
First, let’s define specific heat:
The specific heat of a substance is the heat required in calories to raise the temperature of 1 gram by 1 degree Celsius.
As Fahrenheit continues his measurements of heat flow, it quickly became evident that for different materials, the amount of heat needed to raise the temperature of one gram by one degree could be quite different. For example, it had been widely thought before the measurements were made, that one cc of Mercury, being a lot heavier than one cc of water, would take more heat to raise its temperature by one degree. This proved not to be the case—Fahrenheit himself made the measurement. In an insulating container, called a “calorimeter” he added 100ccs of water at 100°F to 100ccs of mercury at 150°F, and stirred so they quickly reached thermal equilibrium.
Question: what do you think the final temperature was? Approximately?
Answer: The final temperature was, surprisingly, about 120°F. 100 cc of water evidently “contained more heat” than 100 cc of mercury, despite the large difference in weight!
This technique, called calorimetry, was widely used to find the specific heats of many different substances, and at first no clear pattern emerged. It was puzzling that the specific heat of mercury was so low compared with water! As more experiments on different substances were done, it gradually became evident that heavier substances, paradoxically, had lower specific heats.
A Connection With Atomic Theory
Meanwhile, this quantitative approach to scientific observation had spread to chemistry. Towards the end of the 1700’s, Lavoisier weighed chemicals involved in reactions before and after the reaction. This involved weighing the gases involved, so had to be carried out in closed containers, so that, for example, the weight of oxygen used and the carbon dioxide, etc., produced would accounted for in studying combustion. The big discovery was that mass was neither created nor destroyed. This had not been realized before because no one had weighed the gases involved. It made the atomic theory suddenly more plausible, with the idea that maybe chemical reactions were just rearrangements of atoms into different combinations.
Lavoisier also clarified the concept of an element, an idea that was taken up in about 1800 by John Dalton, who argues that a given compound consisted of identical molecules, made up of elementary atoms in the same proportion, such as H2O (although that was thought initially to be HO). This explained why, when substances reacted chemically, such as the burning of hydrogen to form water, it took exactly eight grams of oxygen for each gram of hydrogen. (Well, you could also produce H2O2 under the right conditions, with exactly sixteen grams of oxygen to one of hydrogen, but the simple ratios of amounts of oxygen needed for the two reactions were simply explained by different molecular structures, and made the atomic hypothesis even more plausible.)
Much effort was expended carefully weighing the constituents in many chemical reactions, and constructing diagrams of the molecules. The important result of all this work was that it became possible to list the relative weights of the atoms involved. For example, the data on H2O and H2O2 led to the conclusion that an oxygen atom weighed sixteen times the weight of a hydrogen atom.
It must be emphasized, though, that these results gave no clue as to the actual weights of atoms! All that was known was that atoms were too small to see in the best microscopes. Nevertheless, knowing the relative weights of some atoms in 1820 led to an important discovery. Two professors in France, Dulong and Petit, found that for a whole series of elements the product of atomic weight and specific heat was the same!
The significance of this, as they pointed out, was that the “specific heat”, or heat capacity, of each atom was the same—a piece of lead and a piece of zinc having the same number of atoms would have the same heat capacity. So heavier atoms absorbed no more heat than lighter atoms for a given rise in temperature. This partially explained why mercury had such a surprisingly low heat capacity. Of course, having no idea how big the atoms might be, they could go no further. And, indeed, many of their colleagues didn’t believe in atoms anyway, so it was hard to convince them of the significance of this discovery.
Latent Heat
One of Black’s experiments was to set a pan of water on a steady fire and observe the temperature
as a function of time. He found it steadily increased, reflecting the supply of heat from the fire, until the water began to boil, whereupon the temperature stayed the same for a long time. The steam coming off was at the same (boiling) temperature as the water. So what was happening to the heat being supplied? Black correctly concluded that heat needed to be supplied to change water from its liquid state to its gaseous state, that is, to steam. In fact, a lot of heat had to be supplied: 540 calories per gram, as opposed to the mere 100 calories per gram needed to bring it from the freezing temperature to boiling. He also discovered that it took 80 calories per gram to melt ice into water, with no rise in temperature. This heat is released when the water freezes back to ice, so it is somehow “hidden” in the water. He called it latent heat, meaning hidden heat.
Lectures on Heat (1)
Heat
Feeling and seeing temperature changesWithin some reasonable temperature range, we can get a rough idea how warm something is by touching it. But this can be unreliable—if you put one hand in cold water, one in hot, then plunge both of them into lukewarm water, one hand will tell you it’s hot, the other will feel cold. For something too hot to touch, we can often get an impression of how hot it is by approaching and sensing the radiant heat. If the temperature increases enough, it begins to glow and we can see it’s hot!
The problem with these subjective perceptions of heat is that they may not be the same for everybody. If our two hands can’t agree on whether water is warm or cold, how likely is it that a group of people can set a uniform standard? We need to construct a device of some kind that responds to temperature in a simple, measurable way—we need a thermometer.
The first step on the road to a thermometer was taken by one Philo of Byzantium, an engineer, in the second century BC. He took a hollow lead sphere connected with a tight seal to one end of a pipe, the other end of the pipe being under water in another vessel.
To quote Philo: “…if you expose the sphere to the sun, part of the air enclosed in the tube will pass out when the sphere becomes hot. This will be evident because the air will descend from the tube into the water, agitating it and producing a succession of bubbles.
Now if the sphere is put back in the shade, that is, where the sun’s rays do not reach it, the water will rise and pass through the tube …”
“No matter how many times you repeat the operation, the same thing will happen.
In fact, if you heat the sphere with fire, or even if you pour hot water over it, the result will be the same.”
Notice that Philo did what a real investigative scientist should do—he checked that the experiment was reproducible, and he established that the air’s expansion was in response to heat being applied to the sphere, and was independent of the source of the heat.
Classic Dramatic Uses of Temperature-Dependent Effects
This expansion of air on heating became widely known in classical times, and was used in various dramatic devices. For example, Hero of Alexandria describes a small temple where a fire on the altar causes the doors to open
The altar is a large airtight box, with a pipe leading from it to another enclosed container filled with water. When the fire is set on top of the altar, the air in the box heats up and expands into a second container which is filled with water. This water is forced out through an overflow pipe into a bucket hung on a rope attached to the door hinges in such a way that as the bucket fills with water, it drops, turns the hinges, and opens the doors. The pipe into this bucket reaches almost to the bottom, so that when the altar fire goes out, the water is sucked back and the doors close again. (Presumably, once the fire is burning, the god behind the doors is ready to do business and the doors open…)Still, none of these ingenious devices is a thermometer. There was no attempt (at least none recorded) by Philo or his followers to make a quantitative measurement of how hot or cold the sphere was. And the “meter” in thermometer means measurement
The First Thermometer
Galileo claimed to have invented the first thermometer. Well, actually, he called it a thermoscope, but he did try to measure “degrees of heat and cold” according to a colleague, and that qualifies it as a thermometer. (Technically, a thermoscope is a device making it possible to see a temperature change, a thermometer can measure the temperature change.) Galileo used an inverted narrow-necked bulb with a tubular neck, like a hen’s egg with a long glass tube attached at the tip.
He first heated the bulb with his hands then immediately put it into water. He recorded that the water rose in the bulb the height of “one palm”. Later, either Galileo or his colleague Santorio Santorio put a paper scale next to the tube to read off changes in the water level. This definitely made it a thermometer, but who thought of it first isn’t clear (they argued about it). And, in fact, this thermometer had problems.
Question: what problems? If you occasionally top up the water, why shouldn’t this thermometer be good for recording daily changes in temperature?
Answer: because it’s also a barometer! But—Galileo didn’t know about the atmospheric pressure.
Torricelli, one of Galileo’s pupils, was the first to realize, shortly after Galileo died, that the real driving force in suction was external atmospheric pressure, a satisfying mechanical explanation in contrast to the philosophical “nature abhors a vacuum”. In the 1640’s, Pascal pointed out that the variability of atmospheric pressure rendered the air thermometer untrustworthy.
Liquid-in-glass thermometers were used from the 1630’s, and they were of course insensitive to barometric pressure. Meteorological records were kept from this time, but there was no real uniformity of temperature measurement until Fahrenheit, almost a hundred years later.
Question: what problems? If you occasionally top up the water, why shouldn’t this thermometer be good for recording daily changes in temperature?
Answer: because it’s also a barometer! But—Galileo didn’t know about the atmospheric pressure.
Torricelli, one of Galileo’s pupils, was the first to realize, shortly after Galileo died, that the real driving force in suction was external atmospheric pressure, a satisfying mechanical explanation in contrast to the philosophical “nature abhors a vacuum”. In the 1640’s, Pascal pointed out that the variability of atmospheric pressure rendered the air thermometer untrustworthy.
Liquid-in-glass thermometers were used from the 1630’s, and they were of course insensitive to barometric pressure. Meteorological records were kept from this time, but there was no real uniformity of temperature measurement until Fahrenheit, almost a hundred years later.
Newton’s Anonymous Table of Temperatures
The first systematic account of a range of different temperatures, “Degrees of Heat”, was written by Newton, but published anonymously, in 1701. Presumably he felt that this project lacked the timeless significance of some of his other achievements.
Taking the freezing point of water as zero, Newton found the temperature of boiling water to be almost three times that of the human body, melting lead eight times as great (actually 327C, whereas 8x37=296, so this is pretty good!) but for higher temperatures, such as that of a wood fire, he underestimated considerably. He used a linseed oil liquid in glass thermometer up to the melting point of tin (232°C). (Linseed oil doesn’t boil until 343°C, but that is also its autoignition temperature!)
Newton tried to estimate the higher temperatures indirectly. He heated up a piece of iron in a fire, then let it cool in a steady breeze. He found that, at least at the lower temperatures where he could cross check with his thermometer, the temperature dropped in a geometric progression, that is, if it took five minutes to drop from 80° above air temperature to 40° above air temperature, it took another five minutes to drop to 20° above air, another five to drop to 10° above, and so on. He then assumed this same pattern of temperature drop was true at the high temperatures beyond the reach of his thermometer, and so estimated the temperature of the fire and of iron glowing red hot. This wasn’t very accurate—he (under)estimated the temperature of the fire to be about 600°C.
Fahrenheit’s Excellent Thermometer
The first really good thermometer, using mercury expanding from a bulb into a capillary tube, was made by Fahrenheit in the early 1720’s. He got the idea of using mercury from a colleague’s comment that one should correct a barometer reading to allow for the variation of the density of mercury with temperature. The point that has to be borne in mind in constructing thermometers, and defining temperature scales, is that not all liquids expand at uniform rates on heating—water, for example, at first contracts on heating from its freezing point, then begins to expand at around forty degrees Fahrenheit, so a water thermometer wouldn’t be very helpful on a cold day. It is also not easy to manufacture a uniform cross section capillary tube, but Fahrenheit managed to do it, and demonstrated his success by showing his thermometers agreed with each other over a whole range of temperatures. Fortunately, it turns out that mercury is well behaved in that the temperature scale defined by taking its expansion to be uniform coincides very closely with the true temperature scale, as we shall see later.
Amontons’ Air Thermometer: Pressure Increases Linearly with Temperature
A little earlier (1702) Amontons introduced an air pressure thermometer. He established that if air at atmospheric pressure (he states 30 inches of mercury) at the freezing point of water is enclosed then heated to the boiling point of water, but meanwhile kept at constant volume by increasing the pressure on it, the pressure goes up by about 10 inches of mercury. He also discovered that if he compressed the air in the first place, so that it was at a pressure of sixty inches of mercury at the temperature of melting ice, then if he raised its temperature to that of boiling water, at the same time adding mercury to the column to keep the volume of air constant, the pressure increased by 20 inches of mercury. In other words, he found that for a fixed amount of air kept in a container at constant volume, the pressure increased with temperature by about 33% from freezing to boiling, that percentage being independent of the initial pressure.
The first systematic account of a range of different temperatures, “Degrees of Heat”, was written by Newton, but published anonymously, in 1701. Presumably he felt that this project lacked the timeless significance of some of his other achievements.
Taking the freezing point of water as zero, Newton found the temperature of boiling water to be almost three times that of the human body, melting lead eight times as great (actually 327C, whereas 8x37=296, so this is pretty good!) but for higher temperatures, such as that of a wood fire, he underestimated considerably. He used a linseed oil liquid in glass thermometer up to the melting point of tin (232°C). (Linseed oil doesn’t boil until 343°C, but that is also its autoignition temperature!)
Newton tried to estimate the higher temperatures indirectly. He heated up a piece of iron in a fire, then let it cool in a steady breeze. He found that, at least at the lower temperatures where he could cross check with his thermometer, the temperature dropped in a geometric progression, that is, if it took five minutes to drop from 80° above air temperature to 40° above air temperature, it took another five minutes to drop to 20° above air, another five to drop to 10° above, and so on. He then assumed this same pattern of temperature drop was true at the high temperatures beyond the reach of his thermometer, and so estimated the temperature of the fire and of iron glowing red hot. This wasn’t very accurate—he (under)estimated the temperature of the fire to be about 600°C.
Fahrenheit’s Excellent Thermometer
The first really good thermometer, using mercury expanding from a bulb into a capillary tube, was made by Fahrenheit in the early 1720’s. He got the idea of using mercury from a colleague’s comment that one should correct a barometer reading to allow for the variation of the density of mercury with temperature. The point that has to be borne in mind in constructing thermometers, and defining temperature scales, is that not all liquids expand at uniform rates on heating—water, for example, at first contracts on heating from its freezing point, then begins to expand at around forty degrees Fahrenheit, so a water thermometer wouldn’t be very helpful on a cold day. It is also not easy to manufacture a uniform cross section capillary tube, but Fahrenheit managed to do it, and demonstrated his success by showing his thermometers agreed with each other over a whole range of temperatures. Fortunately, it turns out that mercury is well behaved in that the temperature scale defined by taking its expansion to be uniform coincides very closely with the true temperature scale, as we shall see later.
Amontons’ Air Thermometer: Pressure Increases Linearly with Temperature
A little earlier (1702) Amontons introduced an air pressure thermometer. He established that if air at atmospheric pressure (he states 30 inches of mercury) at the freezing point of water is enclosed then heated to the boiling point of water, but meanwhile kept at constant volume by increasing the pressure on it, the pressure goes up by about 10 inches of mercury. He also discovered that if he compressed the air in the first place, so that it was at a pressure of sixty inches of mercury at the temperature of melting ice, then if he raised its temperature to that of boiling water, at the same time adding mercury to the column to keep the volume of air constant, the pressure increased by 20 inches of mercury. In other words, he found that for a fixed amount of air kept in a container at constant volume, the pressure increased with temperature by about 33% from freezing to boiling, that percentage being independent of the initial pressure.
Definitions of Words and Terms Used in the Gas Processing Industry
absorber
A tower or column that provides contact between natural gas being processed and a liquid solvent.
absorption
The operation in which one or more components in the gas phase are transferred to (absorbed into) a liquid solvent.
absorption factor
A factor which is an indication of the tendency for a given gas phase component to be transferred to the liquid solvent. It is generally expressed as A = L/KV where L and V are the molar flows of liquid and vapor, and K is the average value of the vapor-liquid equilibrium constant for the component of concern.
absorption oil
A hydrocarbon liquid used to absorb and recover components from the natural gas being processed.
acid gas
The hydrogen sulfide and/or carbon dioxide contained in, or extracted from, gas or other streams.
adiabatic expansion
The expansion of a gas, vapor, or liquid stream from a higher pressure to a lower pressure in which there is no heat transfer between the gas, vapor, or liquid and the surroundings.
adsorbent
A solid substance used to remove components from natural gas being processed.
adsorption
The process by which gaseous components are adsorbed on solids because of their molecular attraction to the solid surface.
amine (alkanolamine)
Any of several liquid compounds containing amino nitrogen generally used in water solution to remove, by reversible chemical reaction, hydrogen sulfide and/or carbon dioxide from gas and liquid hydrocarbon streams.
API Gravity
An arbitrary scale expressing the relative density of liquid petroleum products. The scale is calibrated in degrees API, calculated by the following formula:
γ = relative density
associated gas
Gaseous hydrocarbons occuring as a free-gas phase under original oil-reservoir conditions of temperature and pressure.
atmospheric pressure
The pressure exerted on the earth by the earth’s atmosphere. A pressure of 760 mm of mercury, 29.92 inches of mercury, or 14.696 psia is used as a standard for some measurements. State regulatory bodies have set other standards for use in
measuring the legal volume of gas. Atmospheric pressure may also refer to the absolute ambient pressure at any given location.
barrel
A common English-unit mesure of liquid volume which, in the petroleum industry, equals 42 U.S. liquid gallons for petroleum or natural gas liquid products measured at 60°F and equilibrium vapor pressure. One barrel equals 0.159 cubic meters, or 6.29 barrels per cubic meter .
blanket gas
A gas phase maintained in a vessel containing liquid to protect the liquid against air contamination, to reduce the hazard of explosion, or to maintain pressure of the liquid. The source of the gas is external to the vessel.
blow case
A small vessel in which liquid is accumulated and then forced from the vessel by applying gas or air pressure above the liquid level.
blowdown
The act of emptying or depressuring a vessel. This may also refer to discarded material, such as blowdown water from a boiler or cooling tower.
boilaway test
Sometimes used to describe the GPA weathering test for LPgas. Refer to definition for "weathering test".
bottoms
The liquid or residual matter which is withdrawn from the bottom of a fractionator or other vessel during processing or while in storage.
B-P mix
A liquefied hydrocarbon product composed chiefly of butanes and propane. If it originates in a refinery, it may also contain butylenes and propylene. More specifically, it conforms to the GPA specifications for commercial B-P mixes as described in GPA Standard 2140.
breathing
The movement of vapor in or out of an atmospheric pressure storage tank because of a change of level of the stored liquid, a change in the temperature of the vapor space above the liquid, or a change of atmospheric pressure.
bs&w (basic sediment and water)
Waste that collects in the bottom of vessels and tanks containing petroleum or petroleum products.
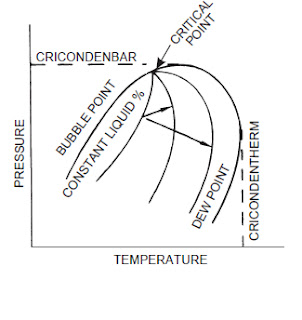
bubble point
The temperature at a specified pressure at which the first stable vapor forms above a liquid.
butane, commercial
A liquefied hydrocarbon consisting predominately of butane and/or butylene and which conforms to the GPA specification for commercial butane defined in GPA Standard 2140.
butane, normal
In commercial transactions, a product meeting the GPA specifications for commercial butane and, in addition, containing a minimum of 95 liquid volume percent normal butane. Chemically, normal butane is an aliphatic compound of the paraffin series having the chemical formula C4H10 and having all of its carbon atoms joined in a straight chain.
calorimeter
An apparatus which is used to determine the heating value of a combustible material.
carbonyl sulfide
A chemical compound of the aldehyde group containing a carbonyl group and sulfur (COS). Sometimes a contaminant in natural gas and NGL. It may need to be removed in order to meet sulfur specifications.
casinghead gas
Unprocessed natural gas produced from a reservoir containing oil. It contains heavier hydrocarbon vapors and is usually produced under low pressure from a casing head on the well.
charcoal test
A test standardized by the Gas Processors Association and the American Gas Association for determining the natural gasoline content of a given natural gas. The gasoline is adsorbed from the gas on activated charcoal and then recovered by distillation.
The test is prescribed in Testing Code 101-43, a joint publication of AGA and GPA.
chromatography
A technique for separating a mixture into individual components by repeated adsorption and desorption on a confined solid bed. It is used for analysis of natural gas and NGL.
Claus Process
A process to convert hydrogen sulfide into elemental sulfur by selective oxidation.
compressibility factor
A factor, usually expressed as "Z," which gives the ratio of the actual volume of gas at a given temperature and pressure to the volume of gas when calculated by the ideal gas law.
compression ratio
The ratio of the absolute discharge pressure from a compressor to the absolute intake pressure. Also applies to one cylinder of a reciprocating compressor and one or more stages of a rotating compressor.
condensate
The liquid formed by the condensation of a vapor or gas; specifically, the hydrocarbon liquid separated from natural gas because of changes in temperature and pressure when the gas
from the reservoir was delivered to the surface separators. In a steam system it may be water that is condensed and returned to the boilers.
convergence pressure
The pressure at a given temperature for a hydrocarbon system of fixed composition at which the vapor-liquid equilibrium Kvalues of the various components in the system become, or
tend to become, unity. The convergence pressure is used to adjust vapor-liquid equilibrium K-values to the particular system under consideration. (See TP-22)
copper strip test
A test using a small strip of pure copper to determine qualitatively the hydrogen sulfide corrosivity of a product. Refer to GPA LP-gas copper strip test (Copper Strip Method), ASTM D-1838 test procedure.
cricondenbar
The highest pressure at which liquid and vapor phases can exist at equilibrium in a multicomponent system.
cricondentherm
The highest temperature at which liquid and vapor phases can exist at equilibrium in a multicomponent system.
critical density
The density of a substance at its critical temperature and critical pressure.
critical pressure
The vapor pressure of a substance at its critical temperature. critical temperature
For a pure component, the maximum temperature at which the component can exist as a liquid.
cryogenic plant
A gas processing plant which is capable of producing natural gas liquid products, including ethane, at very low operating temperatures, usually below minus 50°F.
cubic meter
A unit of volume measurement commonly used in international commerce for petroleum, petroleum products and natural gas. One cubic meter measured at 60°F = 264.172 U.S.
gallons = 6.29 barrels = 35.315 cubic feet measured at 60°F.
deaerator
An item of equipment used for removing air or other non-condensible gases from a process stream or from steam condensate or boiler feed water.
debutanizer
A fractionator designed to separate butane (and more volatile
components if present) from a hydrocarbon mixture.
dehydration
The act or process of removing water from gases or liquids.
demethanized product
A product from which essentially all methane and lighter materials
have been removed.
demethanizer
A fractionator designed to separate methane (and more volatile
components if present) from a hydrocarbon mixture.
depropanizer
A fractionator designed to separate propane (and more volatile
components if present) from a hydrocarbon mixture.
desiccant
A substance used in a dehydrator to remove water and moisture.
Also a material used to remove moisture from the air.
desulfurization
A process by which sulfur and sulfur compounds are removed
from gases or liquid hydrocarbon mixtures.
dew point
The temperature at any given pressure, or the pressure at any
given temperature, at which liquid initially condenses from a
gas or vapor. It is specifically applied to the temperature at
which water vapor starts to condense from a gas mixture
(water dew point), or at which hydrocarbons start to condense
(hydrocarbon dew point).
distillation
The process of separating materials by successively heating to
vaporize a portion and then cooling to liquefy a part of the
vapor. Materials to be separated must differ in boiling point
and/or relative volatility..
doctor test
A qualitative method for detecting hydrogen sulfide and mercaptans
in NGL. The test distinguishes between "sour" and
"sweet" products.
dry gas
(1) Gas whose water content has been reduced by a dehydration
process. (2) Gas containing little or no hydrocarbons commercially
recoverable as liquid product. Gas in this second
definition preferably should be called lean gas.
end point
The maximum temperature observed on the thermometer
during an ASTM distillation test.
EP-mix (ethane-propane mix)
A product consisting of a mixture of essentially ethane and
propane.
expansion turbine
A device which converts part of the energy content of a gas or
liquid stream into mechanical work by expanding the gas or
liquid through a turbine from which work is extracted.
extraction
The process of transferring one or more components from one
liquid phase to another by virtue of different solubility in the
two liquids. It is also used to indicate removal of one or more
constituents from a stream.
field separator
A vessel in the oil or gas field for separating gas, hydrocarbon
liquid, and water from each other.
flash point
The lowest temperature at which vapors from a hydrocarbon
liquid will ignite. See ASTM D-56.
fractionation
See definition of "distillation." Generally used to describe
separation of a mixture of hydrocarbons into individual products
based on difference in boiling point and/or relative volatility.
freeze valve
A specially constructed and calibrated valve designed and
used solely for determining the water content in propane product.
See ASTM D-2713.
gas constant (R)
The constant multiplier in the Ideal Gas Law. Numerically,
R=PV/T, if V is the volume of one mole of an ideal gas at temperature
T and pressure P.
gas hydrate
Refer to definition of "hydrate".
gas injection
The injection of natural gas into a reservoir to maintain or
increase the reservoir pressure or reduce the rate of decline of
the reservoir pressure.
gas lift
A method for bringing crude oil or water to the surface by
injecting gas into the producing well bore.
gas-oil ratio (GOR)
The ratio of gas to liquid hydrocarbon produced from a well.
This may be expressed as standard cubic feet of gas per barrel
of stock tank liquid.
gas processing
The separation of constituents from natural gas for the purpose
of making salable products and also for treating the residue
gas to meet required specifications.
gas processing plant
A plant which processes natural gas for recovery of natural
gas liquids and sometimes other substances such as sulfur.
gas-well gas
The gas produced or separated at surface conditions from the
full well stream produced from a gas reservoir.
gas-well liquids
The liquid separated at surface conditions from the full well
stream produced from a gas reservoir.
gathering system
The network of pipelines which carry gas from the wells to the
processing plant or other separation equipment.
gpm/GPM
(1) gpm (gallons per minute): The term used to describe the
rate of flowing fluid in gallons per minute. (2) GPM — Preferably
Gal/Mcf (gallons per thousand cubic feet): This term refers
to the content in natural gas of components which are
recoverable or recovered as liquid products.
heat medium (heating medium)
A material, whether flowing or static, used to transport heat
from a primary source such as combustion of fuel to another
material. Heating oil, steam, and an eutectic salt mixture are
examples of heat mediums.
heating value (heat of combustion)
The amount of heat obtained by the complete combustion of a
unit quantity of material. The gross, or higher, heating value
is the amount of heat obtained when the water produced in
the combustion is condensed. The net, or lower, heating value
is the amount of heat obtained when the water produced in
the combustion is not condensed.
heavy ends
The portion of a hydrocarbon mixture having the highest boiling
point. Usually hexanes or heptanes and all heavier hydrocarbons
are the heavy ends in a natural gas stream.
hexanes plus (or heptanes plus)
The portion of a hydrocarbon fluid mixture or the last component
of a hydrocarbon analysis which contains the hexanes (or
heptanes) and all hydrocarbons heavier than the hexanes (or
heptanes).
hydrate
A solid material resulting from the combination of a hydrocarbon
with water under pressure.
immiscible
Liquids that will not mix nor blend to give homogeneity are
said to be immiscible.
ideal gas (also called "perfect" gas)
A gas that obeys the ideal gas law expressed as PV=RT, see
Fig. 1-4.
inerts
Elements or compounds not acted upon chemically by the surrounding
environment. Nitrogen and helium are examples of
inert constituents of natural gases.
isobutane
In commercial transactions, a product meeting the GPA specification
for commercial butane and, in addition, containing a
minimum of 95 liquid volume percent isobutane. Chemically,
a hydrocarbon of the paraffin series with the formula C4H10
and having its carbon atoms branched.
jacket water
Water which fills, or is circulated through, a casing which partially
or wholly surrounds a vessel or machine element in order
to remove, add, or distribute heat in order to control the temperature
within the vessel or element.
Joule-Thomson effect
The change in gas temperature which occurs when the gas is
expanded at constant enthalpy from a higher pressure to a
lower pressure. The effect for most gases at normal pressure,
except hydrogen and helium, is a cooling of the gas.
lead acetate test
A method for detecting the presence of hydrogen sulfide by
discoloration of paper which has been moistened with lead acetate
solution. See ASTM D-2420.
lean gas
(1) The residue gas remaining after recovery of natural gas
liquids in a gas processing plant. (2) Unprocessed gas containing
little or no recoverable natural gas liquids.
lean oil
Absorption oil as purchased or recovered by the plant, or oil
from which the absorbed constituents have been removed.
lift gas
Gas used in a gas lift operation.
light ends
The low-boiling, easily evaporated components of a hydrocarbon
liquid mixture.
light hydrocarbons
The low molecular weight hydrocarbons such as methane, ethane,
propane and butanes.
LNG (liquefied natural gas)
The light hydrocarbon portion of natural gas, predominately
methane, which has been liquefied.
loading rack
A structural and piping installation alongside a railroad track
or roadway used for the purpose of filling railroad tank cars
or transport trucks.
LPG (liquefied petroleum gas)
Refer to definition of "LP-gas".
LP-gas (liquefied petroleum gas)
Predominately propane or butane, either separately or in mixtures, which is maintained in a liquid state under pressure within the confining vessel.
LRG (liquefied refinery gas)
Liquid propane or butane produced by a crude oil refinery. It may differ from LP-gas in that propylene and butylene may be present.
LTX (low temperature extraction unit)
A unit which uses the cooling of a constant enthalpy expansion to increase liquid recovery from streams produced from high pressure gas condensate reservoirs. Also called LTS (low temperature separation) unit.
Mcf
An abbreviation for one thousand cubic feet of gas.
MMcf
An abbreviation for one million cubic feet of gas.
mercaptan
Any of a homologous series of compounds of the general formula RSH. All mercaptans possess a foul odor.
miscible flood
A method of secondary recovery of fluids from a reservoir by
injection of fluids that are miscible with the reservoir fluids.
A tower or column that provides contact between natural gas being processed and a liquid solvent.
absorption
The operation in which one or more components in the gas phase are transferred to (absorbed into) a liquid solvent.
absorption factor
A factor which is an indication of the tendency for a given gas phase component to be transferred to the liquid solvent. It is generally expressed as A = L/KV where L and V are the molar flows of liquid and vapor, and K is the average value of the vapor-liquid equilibrium constant for the component of concern.
absorption oil
A hydrocarbon liquid used to absorb and recover components from the natural gas being processed.
acid gas
The hydrogen sulfide and/or carbon dioxide contained in, or extracted from, gas or other streams.
adiabatic expansion
The expansion of a gas, vapor, or liquid stream from a higher pressure to a lower pressure in which there is no heat transfer between the gas, vapor, or liquid and the surroundings.
adsorbent
A solid substance used to remove components from natural gas being processed.
adsorption
The process by which gaseous components are adsorbed on solids because of their molecular attraction to the solid surface.
amine (alkanolamine)
Any of several liquid compounds containing amino nitrogen generally used in water solution to remove, by reversible chemical reaction, hydrogen sulfide and/or carbon dioxide from gas and liquid hydrocarbon streams.
API Gravity
An arbitrary scale expressing the relative density of liquid petroleum products. The scale is calibrated in degrees API, calculated by the following formula:
γ = relative density
associated gas
Gaseous hydrocarbons occuring as a free-gas phase under original oil-reservoir conditions of temperature and pressure.
atmospheric pressure
The pressure exerted on the earth by the earth’s atmosphere. A pressure of 760 mm of mercury, 29.92 inches of mercury, or 14.696 psia is used as a standard for some measurements. State regulatory bodies have set other standards for use in
measuring the legal volume of gas. Atmospheric pressure may also refer to the absolute ambient pressure at any given location.
barrel
A common English-unit mesure of liquid volume which, in the petroleum industry, equals 42 U.S. liquid gallons for petroleum or natural gas liquid products measured at 60°F and equilibrium vapor pressure. One barrel equals 0.159 cubic meters, or 6.29 barrels per cubic meter .
blanket gas
A gas phase maintained in a vessel containing liquid to protect the liquid against air contamination, to reduce the hazard of explosion, or to maintain pressure of the liquid. The source of the gas is external to the vessel.
blow case
A small vessel in which liquid is accumulated and then forced from the vessel by applying gas or air pressure above the liquid level.
blowdown
The act of emptying or depressuring a vessel. This may also refer to discarded material, such as blowdown water from a boiler or cooling tower.
boilaway test
Sometimes used to describe the GPA weathering test for LPgas. Refer to definition for "weathering test".
bottoms
The liquid or residual matter which is withdrawn from the bottom of a fractionator or other vessel during processing or while in storage.
B-P mix
A liquefied hydrocarbon product composed chiefly of butanes and propane. If it originates in a refinery, it may also contain butylenes and propylene. More specifically, it conforms to the GPA specifications for commercial B-P mixes as described in GPA Standard 2140.
breathing
The movement of vapor in or out of an atmospheric pressure storage tank because of a change of level of the stored liquid, a change in the temperature of the vapor space above the liquid, or a change of atmospheric pressure.
bs&w (basic sediment and water)
Waste that collects in the bottom of vessels and tanks containing petroleum or petroleum products.
bubble point
The temperature at a specified pressure at which the first stable vapor forms above a liquid.
butane, commercial
A liquefied hydrocarbon consisting predominately of butane and/or butylene and which conforms to the GPA specification for commercial butane defined in GPA Standard 2140.
butane, normal
In commercial transactions, a product meeting the GPA specifications for commercial butane and, in addition, containing a minimum of 95 liquid volume percent normal butane. Chemically, normal butane is an aliphatic compound of the paraffin series having the chemical formula C4H10 and having all of its carbon atoms joined in a straight chain.
calorimeter
An apparatus which is used to determine the heating value of a combustible material.
carbonyl sulfide
A chemical compound of the aldehyde group containing a carbonyl group and sulfur (COS). Sometimes a contaminant in natural gas and NGL. It may need to be removed in order to meet sulfur specifications.
casinghead gas
Unprocessed natural gas produced from a reservoir containing oil. It contains heavier hydrocarbon vapors and is usually produced under low pressure from a casing head on the well.
charcoal test
A test standardized by the Gas Processors Association and the American Gas Association for determining the natural gasoline content of a given natural gas. The gasoline is adsorbed from the gas on activated charcoal and then recovered by distillation.
The test is prescribed in Testing Code 101-43, a joint publication of AGA and GPA.
chromatography
A technique for separating a mixture into individual components by repeated adsorption and desorption on a confined solid bed. It is used for analysis of natural gas and NGL.
Claus Process
A process to convert hydrogen sulfide into elemental sulfur by selective oxidation.
compressibility factor
A factor, usually expressed as "Z," which gives the ratio of the actual volume of gas at a given temperature and pressure to the volume of gas when calculated by the ideal gas law.
compression ratio
The ratio of the absolute discharge pressure from a compressor to the absolute intake pressure. Also applies to one cylinder of a reciprocating compressor and one or more stages of a rotating compressor.
condensate
The liquid formed by the condensation of a vapor or gas; specifically, the hydrocarbon liquid separated from natural gas because of changes in temperature and pressure when the gas
from the reservoir was delivered to the surface separators. In a steam system it may be water that is condensed and returned to the boilers.
convergence pressure
The pressure at a given temperature for a hydrocarbon system of fixed composition at which the vapor-liquid equilibrium Kvalues of the various components in the system become, or
tend to become, unity. The convergence pressure is used to adjust vapor-liquid equilibrium K-values to the particular system under consideration. (See TP-22)
copper strip test
A test using a small strip of pure copper to determine qualitatively the hydrogen sulfide corrosivity of a product. Refer to GPA LP-gas copper strip test (Copper Strip Method), ASTM D-1838 test procedure.
cricondenbar
The highest pressure at which liquid and vapor phases can exist at equilibrium in a multicomponent system.
cricondentherm
The highest temperature at which liquid and vapor phases can exist at equilibrium in a multicomponent system.
critical density
The density of a substance at its critical temperature and critical pressure.
critical pressure
The vapor pressure of a substance at its critical temperature. critical temperature
For a pure component, the maximum temperature at which the component can exist as a liquid.
cryogenic plant
A gas processing plant which is capable of producing natural gas liquid products, including ethane, at very low operating temperatures, usually below minus 50°F.
cubic meter
A unit of volume measurement commonly used in international commerce for petroleum, petroleum products and natural gas. One cubic meter measured at 60°F = 264.172 U.S.
gallons = 6.29 barrels = 35.315 cubic feet measured at 60°F.
deaerator
An item of equipment used for removing air or other non-condensible gases from a process stream or from steam condensate or boiler feed water.
debutanizer
A fractionator designed to separate butane (and more volatile
components if present) from a hydrocarbon mixture.
dehydration
The act or process of removing water from gases or liquids.
demethanized product
A product from which essentially all methane and lighter materials
have been removed.
demethanizer
A fractionator designed to separate methane (and more volatile
components if present) from a hydrocarbon mixture.
depropanizer
A fractionator designed to separate propane (and more volatile
components if present) from a hydrocarbon mixture.
desiccant
A substance used in a dehydrator to remove water and moisture.
Also a material used to remove moisture from the air.
desulfurization
A process by which sulfur and sulfur compounds are removed
from gases or liquid hydrocarbon mixtures.
dew point
The temperature at any given pressure, or the pressure at any
given temperature, at which liquid initially condenses from a
gas or vapor. It is specifically applied to the temperature at
which water vapor starts to condense from a gas mixture
(water dew point), or at which hydrocarbons start to condense
(hydrocarbon dew point).
distillation
The process of separating materials by successively heating to
vaporize a portion and then cooling to liquefy a part of the
vapor. Materials to be separated must differ in boiling point
and/or relative volatility..
doctor test
A qualitative method for detecting hydrogen sulfide and mercaptans
in NGL. The test distinguishes between "sour" and
"sweet" products.
dry gas
(1) Gas whose water content has been reduced by a dehydration
process. (2) Gas containing little or no hydrocarbons commercially
recoverable as liquid product. Gas in this second
definition preferably should be called lean gas.
end point
The maximum temperature observed on the thermometer
during an ASTM distillation test.
EP-mix (ethane-propane mix)
A product consisting of a mixture of essentially ethane and
propane.
expansion turbine
A device which converts part of the energy content of a gas or
liquid stream into mechanical work by expanding the gas or
liquid through a turbine from which work is extracted.
extraction
The process of transferring one or more components from one
liquid phase to another by virtue of different solubility in the
two liquids. It is also used to indicate removal of one or more
constituents from a stream.
field separator
A vessel in the oil or gas field for separating gas, hydrocarbon
liquid, and water from each other.
flash point
The lowest temperature at which vapors from a hydrocarbon
liquid will ignite. See ASTM D-56.
fractionation
See definition of "distillation." Generally used to describe
separation of a mixture of hydrocarbons into individual products
based on difference in boiling point and/or relative volatility.
freeze valve
A specially constructed and calibrated valve designed and
used solely for determining the water content in propane product.
See ASTM D-2713.
gas constant (R)
The constant multiplier in the Ideal Gas Law. Numerically,
R=PV/T, if V is the volume of one mole of an ideal gas at temperature
T and pressure P.
gas hydrate
Refer to definition of "hydrate".
gas injection
The injection of natural gas into a reservoir to maintain or
increase the reservoir pressure or reduce the rate of decline of
the reservoir pressure.
gas lift
A method for bringing crude oil or water to the surface by
injecting gas into the producing well bore.
gas-oil ratio (GOR)
The ratio of gas to liquid hydrocarbon produced from a well.
This may be expressed as standard cubic feet of gas per barrel
of stock tank liquid.
gas processing
The separation of constituents from natural gas for the purpose
of making salable products and also for treating the residue
gas to meet required specifications.
gas processing plant
A plant which processes natural gas for recovery of natural
gas liquids and sometimes other substances such as sulfur.
gas-well gas
The gas produced or separated at surface conditions from the
full well stream produced from a gas reservoir.
gas-well liquids
The liquid separated at surface conditions from the full well
stream produced from a gas reservoir.
gathering system
The network of pipelines which carry gas from the wells to the
processing plant or other separation equipment.
gpm/GPM
(1) gpm (gallons per minute): The term used to describe the
rate of flowing fluid in gallons per minute. (2) GPM — Preferably
Gal/Mcf (gallons per thousand cubic feet): This term refers
to the content in natural gas of components which are
recoverable or recovered as liquid products.
heat medium (heating medium)
A material, whether flowing or static, used to transport heat
from a primary source such as combustion of fuel to another
material. Heating oil, steam, and an eutectic salt mixture are
examples of heat mediums.
heating value (heat of combustion)
The amount of heat obtained by the complete combustion of a
unit quantity of material. The gross, or higher, heating value
is the amount of heat obtained when the water produced in
the combustion is condensed. The net, or lower, heating value
is the amount of heat obtained when the water produced in
the combustion is not condensed.
heavy ends
The portion of a hydrocarbon mixture having the highest boiling
point. Usually hexanes or heptanes and all heavier hydrocarbons
are the heavy ends in a natural gas stream.
hexanes plus (or heptanes plus)
The portion of a hydrocarbon fluid mixture or the last component
of a hydrocarbon analysis which contains the hexanes (or
heptanes) and all hydrocarbons heavier than the hexanes (or
heptanes).
hydrate
A solid material resulting from the combination of a hydrocarbon
with water under pressure.
immiscible
Liquids that will not mix nor blend to give homogeneity are
said to be immiscible.
ideal gas (also called "perfect" gas)
A gas that obeys the ideal gas law expressed as PV=RT, see
Fig. 1-4.
inerts
Elements or compounds not acted upon chemically by the surrounding
environment. Nitrogen and helium are examples of
inert constituents of natural gases.
isobutane
In commercial transactions, a product meeting the GPA specification
for commercial butane and, in addition, containing a
minimum of 95 liquid volume percent isobutane. Chemically,
a hydrocarbon of the paraffin series with the formula C4H10
and having its carbon atoms branched.
jacket water
Water which fills, or is circulated through, a casing which partially
or wholly surrounds a vessel or machine element in order
to remove, add, or distribute heat in order to control the temperature
within the vessel or element.
Joule-Thomson effect
The change in gas temperature which occurs when the gas is
expanded at constant enthalpy from a higher pressure to a
lower pressure. The effect for most gases at normal pressure,
except hydrogen and helium, is a cooling of the gas.
lead acetate test
A method for detecting the presence of hydrogen sulfide by
discoloration of paper which has been moistened with lead acetate
solution. See ASTM D-2420.
lean gas
(1) The residue gas remaining after recovery of natural gas
liquids in a gas processing plant. (2) Unprocessed gas containing
little or no recoverable natural gas liquids.
lean oil
Absorption oil as purchased or recovered by the plant, or oil
from which the absorbed constituents have been removed.
lift gas
Gas used in a gas lift operation.
light ends
The low-boiling, easily evaporated components of a hydrocarbon
liquid mixture.
light hydrocarbons
The low molecular weight hydrocarbons such as methane, ethane,
propane and butanes.
LNG (liquefied natural gas)
The light hydrocarbon portion of natural gas, predominately
methane, which has been liquefied.
loading rack
A structural and piping installation alongside a railroad track
or roadway used for the purpose of filling railroad tank cars
or transport trucks.
LPG (liquefied petroleum gas)
Refer to definition of "LP-gas".
LP-gas (liquefied petroleum gas)
Predominately propane or butane, either separately or in mixtures, which is maintained in a liquid state under pressure within the confining vessel.
LRG (liquefied refinery gas)
Liquid propane or butane produced by a crude oil refinery. It may differ from LP-gas in that propylene and butylene may be present.
LTX (low temperature extraction unit)
A unit which uses the cooling of a constant enthalpy expansion to increase liquid recovery from streams produced from high pressure gas condensate reservoirs. Also called LTS (low temperature separation) unit.
Mcf
An abbreviation for one thousand cubic feet of gas.
MMcf
An abbreviation for one million cubic feet of gas.
mercaptan
Any of a homologous series of compounds of the general formula RSH. All mercaptans possess a foul odor.
miscible flood
A method of secondary recovery of fluids from a reservoir by
injection of fluids that are miscible with the reservoir fluids.
Subscribe to:
Posts (Atom)