Introduction
While this book is not
intended as a comprehensive course in electricity and magnetism
there are a few
principles that are so ubiquitous in functional magnetic resonance that
they appear repeatedly
throughout this text and therefore warrant this brief overview.
Charge
Electrical charge is
considered to be a fundamental property of materials. Physicists
recognize that charge
exists in only two forms, positive and negative, and that it is
quantal in nature,
with the smallest amount of charge being that of a single electron or
proton, each being
exactly 1 unit of negative or positive charge, respectively. A single
unit of charge is
extremely small, of course, and charge is more commonly measured in
units of Coulombs, equivalent to about
6.242 X 1018 unit
charges. Positive and negative
charges exhibit a
strong attractive force, whose magnitude is proportional inversely to
the square root of the
distance that separates them. In its most stable state, bulk matter
has a net charge of zero, meaning that it contains an
identical number of positive and
negative charges.
Voltage
When charges become
separated by distance, the presence of an attractive force between
implies an increase in
potential energy, which is released when the charges are moved
together. This energy
difference is known as Voltage and is measured, naturally, in
Volts. Because the
potential energy of the Voltage is also measure of the force that would
tend to move the
charge, it is known also as the potential difference, or simply the
potential, the “electromotive
force” or the e.m.f. and
these terms are used
interchangeably, which
can at times be confusing. Batteries are familiar voltage sources
that rely on chemical
means to store potential energy. For convenience, the units of
Volts are defined in
terms of other fundamental physical constants and units. One
Joule of work is required to move one Coulomb
of charge through a potential difference
of 1 Volt. In
practice, this means that a Coulomb is actually defined to set unit values of
Volts and Joules.
Voltage must always refer to the energy difference
between two points.
It is never actually
correct to discuss the Voltage at a point, though you will often see
such a statement. In
those cases, the reference point is assumed implicitly, usually to
refer to a “ground” or
common point in an electrical circuit.
At the atomic level
charges may become separated. In some molecules, such as salts like
sodium chloride, the electronegativity of one
atom (chloride) is so much greater than
that of the other
(sodium) that in a covalent atomic bond between these elements the
electron or electrons
are almost completely transferred from one atom to the other.
Such bonds are
dissociated easily in aqueous solution so that the individual atoms now
become “ions” or charged particles. In water, the atoms of
salts appear in ionic form, so
that atoms of sodium,
potassium, chloride, magnesium and many others move relatively
freely of their
oppositely charged complement. Not only atoms, but also molecules, can
exist in ionic form,
and many proteins, for example, carry a net negative charge. Of
course some ions may
be quite large and there may be physical impediments to their
motion that result in
different bulk properties for ions and small charges, such as
electrons. These
effects are significant in some circumstances, but in most of the
discussion that follows, and throughout most of this book, we can consider the
properties of ions interchangeably with the properties of charge.
Current and Resistance
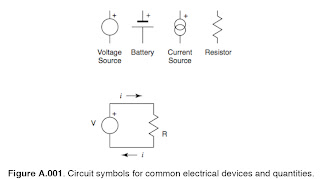
The motion of charge is known as current; specifically, the current, i, is equal to the
change in charge, Q, with time, so that:
Where V is the Voltage, i is the current, and R the resistance. Materials whose resistance
is extremely high are termed insulators and those whose resistance is low are called,
conductors. Good insulators may have resistance of gigaOhms (109 Ohms) or more,
whereas good conductors, such as copper wire, will have resistance of microOhms. More
accurately, we refer to resistivity, which is the measured resistance normalized by the
area and length of a conductor, so that it is a material property. Most biological
materials fall in a more intermediate range with resistances of thousands to millions of
Ohms. In a perfect conductor, where the resistance is zero, the voltage at all points along
the conductor is identical. In general, moving charge from a source of higher potential
energy to lower (current flowing from positive to negative ends of a source) must result
in energy dissipation. Resistors dissipate this energy as heat.