Freezing is the unit operation in which the temperature of a food is reduced below its
freezing point and a proportion of the water undergoes a change in state to form ice
crystals. The immobilisation of water to ice and the resulting concentration of dissolved
solutes in unfrozen water lower the water activity (aw) of the food (aw is described in
Chapter 1). Preservation is achieved by a combination of low temperatures, reduced
water activity and, in some foods, pre-treatment by blanching. There are only small
changes to nutritional or sensory qualities of foods when correct freezing and storage
procedures are followed.
The major groups of commercially frozen foods are as follows:
• fruits (strawberries, oranges, raspberries, blackcurrants) either whole or pure´ed, or as
juice concentrates
• vegetables (peas, green beans, sweetcorn, spinach, sprouts and potatoes)
• fish fillets and seafoods (cod, plaice, shrimps and crab meat) including fish fingers,
fish cakes or prepared dishes with an accompanying sauce
• meats (beef, lamb, poultry) as carcasses, boxed joints or cubes, and meat products
(sausages, beefburgers, reformed steaks)
• baked goods (bread, cakes, fruit and meat pies)
• prepared foods (pizzas, desserts, ice cream, complete meals and cook–freeze dishes).
Rapid increases in sales of frozen foods in recent years are closely associated with
increased ownership of domestic freezers and microwave ovens. Frozen foods and chilled
foods (Chapter 19) have an image of high quality and ‘freshness’ and, particularly in
meat, fruit and vegetable sectors, outsell canned or dried products.
Distribution of frozen foods has a relatively high cost, due to the need to maintain a
constant low temperature. Distribution logistics are discussed further in Chapter 19 in
relation to chilled foods and in Chapter 26. A recent advance in distribution of chilled
and frozen foods is described by Jennings (1999), in which carbon dioxide ‘snow’
(Section 21.2.4) is added to sealed containers of food, which are then loaded into
normal distribution vehicles. The time that a product can be held at the required chilled
or frozen storage temperature can be varied from four to 24 hours by adjusting the
amount of added snow. Other advantages of the system include greater flexibility in
being able to carry mixed loads at different temperatures in the same vehicle, greater
control over storage temperature and greater flexibility in use, compared to standard
refrigerated vehicles.
21.1 Theory
During freezing, sensible heat is first removed to lower the temperature of a food to the
freezing point. In fresh foods, heat produced by respiration is also removed (Chapter 19).
This is termed the heat load, and is important in determining the correct size of freezing
equipment for a particular production rate. Most foods contain a large proportion of water
(Table 21.1), which has a high specific heat (4200 J kg 1 K 1) and a high latent heat of
crystallisation (335 kJ kg 1). A substantial amount of energy is therefore needed to
remove latent heat, form ice crystals and hence to freeze foods. The latent heat of other
components of the food (for example fats) must also be removed before they can solidify
but in most foods these other components are present in smaller amounts and removal of
a relatively small amount of heat is needed for crystallisation to take place. Energy for
freezing is supplied as electrical energy, which is used to compress gases (refrigerants) in
mechanical freezing equipment (Sections 21.2.1–3) or to compress and cool cryogens
(Section 21.2.4).
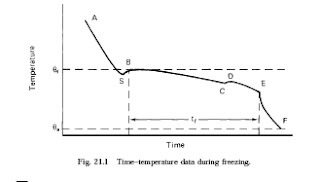
If the temperature is monitored at the thermal centre of a food (the point that cools
most slowly) as heat is removed, a characteristic curve is obtained (Fig. 21.1).
The six components of the curve are as follows.
AS The food is cooled to below its freezing point f which, with the exception of
pure water, is always below 0ºC (Table 21.1). At point S the water remains
liquid, although the temperature is below the freezing point. This phenomenon is
known as supercooling and may be as much as 10ºC below the freezing point.
SB The temperature rises rapidly to the freezing point as ice crystals begin to form
and latent heat of crystallisation is released.
BC Heat is removed from the food at the same rate as before, but it is latent heat
being removed as ice forms and the temperature therefore remains almost
constant. The freezing point is gradually depressed by the increase in solute
concentration in the unfrozen liquor, and the temperature therefore falls slightly.
It is during this stage that the major part of the ice is formed (Fig. 21.2).
CD One of the solutes becomes supersaturated and crystallises out. The latent heat
of crystallisation is released and the temperature rises to the eutectic temperature
for that solute (Section 21.1.2).
DE Crystallisation of water and solutes continues. The total time tf taken (the
freezing plateau) is determined by the rate at which heat is removed.
EF The temperature of the ice–water mixture falls to the temperature of the freezer.
A proportion of the water remains unfrozen at the temperatures used in
commercial freezing; the amount depends on the type and composition of the
food and the temperature of storage. For example at a storage temperature of
20ºC the percentage of water frozen is 88% in lamb, 91% in fish and 93% in
egg albumin.
freezing point and a proportion of the water undergoes a change in state to form ice
crystals. The immobilisation of water to ice and the resulting concentration of dissolved
solutes in unfrozen water lower the water activity (aw) of the food (aw is described in
Chapter 1). Preservation is achieved by a combination of low temperatures, reduced
water activity and, in some foods, pre-treatment by blanching. There are only small
changes to nutritional or sensory qualities of foods when correct freezing and storage
procedures are followed.
The major groups of commercially frozen foods are as follows:
• fruits (strawberries, oranges, raspberries, blackcurrants) either whole or pure´ed, or as
juice concentrates
• vegetables (peas, green beans, sweetcorn, spinach, sprouts and potatoes)
• fish fillets and seafoods (cod, plaice, shrimps and crab meat) including fish fingers,
fish cakes or prepared dishes with an accompanying sauce
• meats (beef, lamb, poultry) as carcasses, boxed joints or cubes, and meat products
(sausages, beefburgers, reformed steaks)
• baked goods (bread, cakes, fruit and meat pies)
• prepared foods (pizzas, desserts, ice cream, complete meals and cook–freeze dishes).
Rapid increases in sales of frozen foods in recent years are closely associated with
increased ownership of domestic freezers and microwave ovens. Frozen foods and chilled
foods (Chapter 19) have an image of high quality and ‘freshness’ and, particularly in
meat, fruit and vegetable sectors, outsell canned or dried products.
Distribution of frozen foods has a relatively high cost, due to the need to maintain a
constant low temperature. Distribution logistics are discussed further in Chapter 19 in
relation to chilled foods and in Chapter 26. A recent advance in distribution of chilled
and frozen foods is described by Jennings (1999), in which carbon dioxide ‘snow’
(Section 21.2.4) is added to sealed containers of food, which are then loaded into
normal distribution vehicles. The time that a product can be held at the required chilled
or frozen storage temperature can be varied from four to 24 hours by adjusting the
amount of added snow. Other advantages of the system include greater flexibility in
being able to carry mixed loads at different temperatures in the same vehicle, greater
control over storage temperature and greater flexibility in use, compared to standard
refrigerated vehicles.
21.1 Theory
During freezing, sensible heat is first removed to lower the temperature of a food to the
freezing point. In fresh foods, heat produced by respiration is also removed (Chapter 19).
This is termed the heat load, and is important in determining the correct size of freezing
equipment for a particular production rate. Most foods contain a large proportion of water
(Table 21.1), which has a high specific heat (4200 J kg 1 K 1) and a high latent heat of
crystallisation (335 kJ kg 1). A substantial amount of energy is therefore needed to
remove latent heat, form ice crystals and hence to freeze foods. The latent heat of other
components of the food (for example fats) must also be removed before they can solidify
but in most foods these other components are present in smaller amounts and removal of
a relatively small amount of heat is needed for crystallisation to take place. Energy for
freezing is supplied as electrical energy, which is used to compress gases (refrigerants) in
mechanical freezing equipment (Sections 21.2.1–3) or to compress and cool cryogens
(Section 21.2.4).
If the temperature is monitored at the thermal centre of a food (the point that cools
most slowly) as heat is removed, a characteristic curve is obtained (Fig. 21.1).
The six components of the curve are as follows.
AS The food is cooled to below its freezing point f which, with the exception of
pure water, is always below 0ºC (Table 21.1). At point S the water remains
liquid, although the temperature is below the freezing point. This phenomenon is
known as supercooling and may be as much as 10ºC below the freezing point.
SB The temperature rises rapidly to the freezing point as ice crystals begin to form
and latent heat of crystallisation is released.
BC Heat is removed from the food at the same rate as before, but it is latent heat
being removed as ice forms and the temperature therefore remains almost
constant. The freezing point is gradually depressed by the increase in solute
concentration in the unfrozen liquor, and the temperature therefore falls slightly.
It is during this stage that the major part of the ice is formed (Fig. 21.2).
CD One of the solutes becomes supersaturated and crystallises out. The latent heat
of crystallisation is released and the temperature rises to the eutectic temperature
for that solute (Section 21.1.2).
DE Crystallisation of water and solutes continues. The total time tf taken (the
freezing plateau) is determined by the rate at which heat is removed.
EF The temperature of the ice–water mixture falls to the temperature of the freezer.
A proportion of the water remains unfrozen at the temperatures used in
commercial freezing; the amount depends on the type and composition of the
food and the temperature of storage. For example at a storage temperature of
20ºC the percentage of water frozen is 88% in lamb, 91% in fish and 93% in
egg albumin.