absorberA tower or column that provides contact between natural gas being processed and a liquid solvent.
absorptionThe operation in which one or more components in the gas phase are transferred to (absorbed into) a liquid solvent.
absorption factorA factor which is an indication of the tendency for a given gas phase component to be transferred to the liquid solvent. It is generally expressed as A = L/KV where L and V are the molar flows of liquid and vapor, and K is the average value of the vapor-liquid equilibrium constant for the component of concern.
absorption oilA hydrocarbon liquid used to absorb and recover components from the natural gas being processed.
acid gasThe hydrogen sulfide and/or carbon dioxide contained in, or extracted from, gas or other streams.
adiabatic expansionThe expansion of a gas, vapor, or liquid stream from a higher pressure to a lower pressure in which there is no heat transfer between the gas, vapor, or liquid and the surroundings.
adsorbentA solid substance used to remove components from natural gas being processed.
adsorptionThe process by which gaseous components are adsorbed on solids because of their molecular attraction to the solid surface.
amine (alkanolamine)Any of several liquid compounds containing amino nitrogen generally used in water solution to remove, by reversible chemical reaction, hydrogen sulfide and/or carbon dioxide from gas and liquid hydrocarbon streams.
API GravityAn arbitrary scale expressing the relative density of liquid petroleum products. The scale is calibrated in degrees API, calculated by the following formula:
γ = relative density
associated gasGaseous hydrocarbons occuring as a free-gas phase under original oil-reservoir conditions of temperature and pressure.
atmospheric pressureThe pressure exerted on the earth by the earth’s atmosphere. A pressure of 760 mm of mercury, 29.92 inches of mercury, or 14.696 psia is used as a standard for some measurements. State regulatory bodies have set other standards for use in
measuring the legal volume of gas. Atmospheric pressure may also refer to the absolute ambient pressure at any given location.
barrelA common English-unit mesure of liquid volume which, in the petroleum industry, equals 42 U.S. liquid gallons for petroleum or natural gas liquid products measured at 60°F and equilibrium vapor pressure. One barrel equals 0.159 cubic meters, or 6.29 barrels per cubic meter .
blanket gasA gas phase maintained in a vessel containing liquid to protect the liquid against air contamination, to reduce the hazard of explosion, or to maintain pressure of the liquid. The source of the gas is external to the vessel.
blow caseA small vessel in which liquid is accumulated and then forced from the vessel by applying gas or air pressure above the liquid level.
blowdownThe act of emptying or depressuring a vessel. This may also refer to discarded material, such as blowdown water from a boiler or cooling tower.
boilaway testSometimes used to describe the GPA weathering test for LPgas. Refer to definition for "weathering test".
bottomsThe liquid or residual matter which is withdrawn from the bottom of a fractionator or other vessel during processing or while in storage.
B-P mixA liquefied hydrocarbon product composed chiefly of butanes and propane. If it originates in a refinery, it may also contain butylenes and propylene. More specifically, it conforms to the GPA specifications for commercial B-P mixes as described in GPA Standard 2140.
breathingThe movement of vapor in or out of an atmospheric pressure storage tank because of a change of level of the stored liquid, a change in the temperature of the vapor space above the liquid, or a change of atmospheric pressure.
bs&w (basic sediment and water)Waste that collects in the bottom of vessels and tanks containing petroleum or petroleum products.
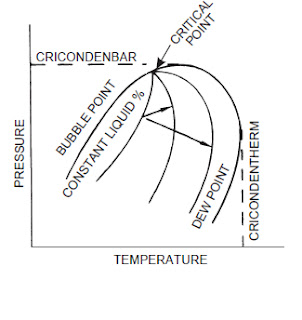
bubble pointThe temperature at a specified pressure at which the first stable vapor forms above a liquid.
butane, commercialA liquefied hydrocarbon consisting predominately of butane and/or butylene and which conforms to the GPA specification for commercial butane defined in GPA Standard 2140.
butane, normalIn commercial transactions, a product meeting the GPA specifications for commercial butane and, in addition, containing a minimum of 95 liquid volume percent normal butane. Chemically, normal butane is an aliphatic compound of the paraffin series having the chemical formula C4H10 and having all of its carbon atoms joined in a straight chain.
calorimeterAn apparatus which is used to determine the heating value of a combustible material.
carbonyl sulfideA chemical compound of the aldehyde group containing a carbonyl group and sulfur (COS). Sometimes a contaminant in natural gas and NGL. It may need to be removed in order to meet sulfur specifications.
casinghead gasUnprocessed natural gas produced from a reservoir containing oil. It contains heavier hydrocarbon vapors and is usually produced under low pressure from a casing head on the well.
charcoal testA test standardized by the Gas Processors Association and the American Gas Association for determining the natural gasoline content of a given natural gas. The gasoline is adsorbed from the gas on activated charcoal and then recovered by distillation.
The test is prescribed in Testing Code 101-43, a joint publication of AGA and GPA.
chromatographyA technique for separating a mixture into individual components by repeated adsorption and desorption on a confined solid bed. It is used for analysis of natural gas and NGL.
Claus ProcessA process to convert hydrogen sulfide into elemental sulfur by selective oxidation.
compressibility factorA factor, usually expressed as "Z," which gives the ratio of the actual volume of gas at a given temperature and pressure to the volume of gas when calculated by the ideal gas law.
compression ratioThe ratio of the absolute discharge pressure from a compressor to the absolute intake pressure. Also applies to one cylinder of a reciprocating compressor and one or more stages of a rotating compressor.
condensateThe liquid formed by the condensation of a vapor or gas; specifically, the hydrocarbon liquid separated from natural gas because of changes in temperature and pressure when the gas
from the reservoir was delivered to the surface separators. In a steam system it may be water that is condensed and returned to the boilers.
convergence pressureThe pressure at a given temperature for a hydrocarbon system of fixed composition at which the vapor-liquid equilibrium Kvalues of the various components in the system become, or
tend to become, unity. The convergence pressure is used to adjust vapor-liquid equilibrium K-values to the particular system under consideration. (See TP-22)
copper strip testA test using a small strip of pure copper to determine qualitatively the hydrogen sulfide corrosivity of a product. Refer to GPA LP-gas copper strip test (Copper Strip Method), ASTM D-1838 test procedure.
cricondenbarThe highest pressure at which liquid and vapor phases can exist at equilibrium in a multicomponent system.
cricondenthermThe highest temperature at which liquid and vapor phases can exist at equilibrium in a multicomponent system.
critical densityThe density of a substance at its critical temperature and critical pressure.
critical pressureThe vapor pressure of a substance at its critical temperature. critical temperature
For a pure component, the maximum temperature at which the component can exist as a liquid.
cryogenic plantA gas processing plant which is capable of producing natural gas liquid products, including ethane, at very low operating temperatures, usually below minus 50°F.
cubic meterA unit of volume measurement commonly used in international commerce for petroleum, petroleum products and natural gas. One cubic meter measured at 60°F = 264.172 U.S.
gallons = 6.29 barrels = 35.315 cubic feet measured at 60°F.
deaeratorAn item of equipment used for removing air or other non-condensible gases from a process stream or from steam condensate or boiler feed water.
debutanizerA fractionator designed to separate butane (and more volatile
components if present) from a hydrocarbon mixture.
dehydrationThe act or process of removing water from gases or liquids.
demethanized product
A product from which essentially all methane and lighter materials
have been removed.
demethanizerA fractionator designed to separate methane (and more volatile
components if present) from a hydrocarbon mixture.
depropanizerA fractionator designed to separate propane (and more volatile
components if present) from a hydrocarbon mixture.
desiccantA substance used in a dehydrator to remove water and moisture.
Also a material used to remove moisture from the air.
desulfurizationA process by which sulfur and sulfur compounds are removed
from gases or liquid hydrocarbon mixtures.
dew pointThe temperature at any given pressure, or the pressure at any
given temperature, at which liquid initially condenses from a
gas or vapor. It is specifically applied to the temperature at
which water vapor starts to condense from a gas mixture
(water dew point), or at which hydrocarbons start to condense
(hydrocarbon dew point).
distillationThe process of separating materials by successively heating to
vaporize a portion and then cooling to liquefy a part of the
vapor. Materials to be separated must differ in boiling point
and/or relative volatility..
doctor testA qualitative method for detecting hydrogen sulfide and mercaptans
in NGL. The test distinguishes between "sour" and
"sweet" products.
dry gas(1) Gas whose water content has been reduced by a dehydration
process. (2) Gas containing little or no hydrocarbons commercially
recoverable as liquid product. Gas in this second
definition preferably should be called lean gas.
end pointThe maximum temperature observed on the thermometer
during an ASTM distillation test.
EP-mix (ethane-propane mix)
A product consisting of a mixture of essentially ethane and
propane.
expansion turbineA device which converts part of the energy content of a gas or
liquid stream into mechanical work by expanding the gas or
liquid through a turbine from which work is extracted.
extractionThe process of transferring one or more components from one
liquid phase to another by virtue of different solubility in the
two liquids. It is also used to indicate removal of one or more
constituents from a stream.
field separatorA vessel in the oil or gas field for separating gas, hydrocarbon
liquid, and water from each other.
flash pointThe lowest temperature at which vapors from a hydrocarbon
liquid will ignite. See ASTM D-56.
fractionationSee definition of "distillation." Generally used to describe
separation of a mixture of hydrocarbons into individual products
based on difference in boiling point and/or relative volatility.
freeze valveA specially constructed and calibrated valve designed and
used solely for determining the water content in propane product.
See ASTM D-2713.
gas constant (R)The constant multiplier in the Ideal Gas Law. Numerically,
R=PV/T, if V is the volume of one mole of an ideal gas at temperature
T and pressure P.
gas hydrateRefer to definition of "hydrate".
gas injectionThe injection of natural gas into a reservoir to maintain or
increase the reservoir pressure or reduce the rate of decline of
the reservoir pressure.
gas liftA method for bringing crude oil or water to the surface by
injecting gas into the producing well bore.
gas-oil ratio (GOR)The ratio of gas to liquid hydrocarbon produced from a well.
This may be expressed as standard cubic feet of gas per barrel
of stock tank liquid.
gas processingThe separation of constituents from natural gas for the purpose
of making salable products and also for treating the residue
gas to meet required specifications.
gas processing plantA plant which processes natural gas for recovery of natural
gas liquids and sometimes other substances such as sulfur.
gas-well gasThe gas produced or separated at surface conditions from the
full well stream produced from a gas reservoir.
gas-well liquidsThe liquid separated at surface conditions from the full well
stream produced from a gas reservoir.
gathering systemThe network of pipelines which carry gas from the wells to the
processing plant or other separation equipment.
gpm/GPM(1) gpm (gallons per minute): The term used to describe the
rate of flowing fluid in gallons per minute. (2) GPM — Preferably
Gal/Mcf (gallons per thousand cubic feet): This term refers
to the content in natural gas of components which are
recoverable or recovered as liquid products.
heat medium (heating medium)A material, whether flowing or static, used to transport heat
from a primary source such as combustion of fuel to another
material. Heating oil, steam, and an eutectic salt mixture are
examples of heat mediums.
heating value (heat of combustion)The amount of heat obtained by the complete combustion of a
unit quantity of material. The gross, or higher, heating value
is the amount of heat obtained when the water produced in
the combustion is condensed. The net, or lower, heating value
is the amount of heat obtained when the water produced in
the combustion is not condensed.
heavy endsThe portion of a hydrocarbon mixture having the highest boiling
point. Usually hexanes or heptanes and all heavier hydrocarbons
are the heavy ends in a natural gas stream.
hexanes plus (or heptanes plus)The portion of a hydrocarbon fluid mixture or the last component
of a hydrocarbon analysis which contains the hexanes (or
heptanes) and all hydrocarbons heavier than the hexanes (or
heptanes).
hydrateA solid material resulting from the combination of a hydrocarbon
with water under pressure.
immiscibleLiquids that will not mix nor blend to give homogeneity are
said to be immiscible.
ideal gas (also called "perfect" gas)
A gas that obeys the ideal gas law expressed as PV=RT, see
Fig. 1-4.
inertsElements or compounds not acted upon chemically by the surrounding
environment. Nitrogen and helium are examples of
inert constituents of natural gases.
isobutaneIn commercial transactions, a product meeting the GPA specification
for commercial butane and, in addition, containing a
minimum of 95 liquid volume percent isobutane. Chemically,
a hydrocarbon of the paraffin series with the formula C4H10
and having its carbon atoms branched.
jacket waterWater which fills, or is circulated through, a casing which partially
or wholly surrounds a vessel or machine element in order
to remove, add, or distribute heat in order to control the temperature
within the vessel or element.
Joule-Thomson effectThe change in gas temperature which occurs when the gas is
expanded at constant enthalpy from a higher pressure to a
lower pressure. The effect for most gases at normal pressure,
except hydrogen and helium, is a cooling of the gas.
lead acetate testA method for detecting the presence of hydrogen sulfide by
discoloration of paper which has been moistened with lead acetate
solution. See ASTM D-2420.
lean gas(1) The residue gas remaining after recovery of natural gas
liquids in a gas processing plant. (2) Unprocessed gas containing
little or no recoverable natural gas liquids.
lean oilAbsorption oil as purchased or recovered by the plant, or oil
from which the absorbed constituents have been removed.
lift gasGas used in a gas lift operation.
light endsThe low-boiling, easily evaporated components of a hydrocarbon
liquid mixture.
light hydrocarbonsThe low molecular weight hydrocarbons such as methane, ethane,
propane and butanes.
LNG (liquefied natural gas)The light hydrocarbon portion of natural gas, predominately
methane, which has been liquefied.
loading rackA structural and piping installation alongside a railroad track
or roadway used for the purpose of filling railroad tank cars
or transport trucks.
LPG (liquefied petroleum gas)Refer to definition of "LP-gas".
LP-gas (liquefied petroleum gas)Predominately propane or butane, either separately or in mixtures, which is maintained in a liquid state under pressure within the confining vessel.
LRG (liquefied refinery gas)Liquid propane or butane produced by a crude oil refinery. It may differ from LP-gas in that propylene and butylene may be present.
LTX (low temperature extraction unit)A unit which uses the cooling of a constant enthalpy expansion to increase liquid recovery from streams produced from high pressure gas condensate reservoirs. Also called LTS (low temperature separation) unit.
McfAn abbreviation for one thousand cubic feet of gas.
MMcfAn abbreviation for one million cubic feet of gas.
mercaptanAny of a homologous series of compounds of the general formula RSH. All mercaptans possess a foul odor.
miscible floodA method of secondary recovery of fluids from a reservoir by
injection of fluids that are miscible with the reservoir fluids.