Corrosion
Corrosion is defined as destruction of a metal by chemical or electrochemical reaction with its environment.’ It is reported that 80% of failures in production and pipeline operations are caused by corrosion.
Corrosion may be apparent by metal loss, strength loss by cracking and solids problems
caused by formation of corrosion by-products. One of the first decisions in well completion design is the selection of the proper casing and tubing. Corrosion will not alter calculation of the tensile, burst and collapse, but it may dictate the selection of the grade of material necessary to satisfy these requirements. Corrosion is common in almost all hydrocarbon-producing environments and costs hundreds of millions of dollars throughout the industry every year.3 In addition, the economic problems are intensified through loss of revenues due to down time and deferred production. For corrosion to occur, there must be a circuit produced through which electrical current can flow. The circuit is called the corrosion cell and the electrical current produced by the process, although very small, can do enormous damage to metal systems. The basic cause of corrosion is instability of metal in its refined form. Because of the free energy relationship, the metals tend to revert to their natural state through the process of corrosion. Pure metals rarely exist in the natural world. To obtain a pure metal, a salt of the metal (the ore) is refined (energy added). This energy input is stored in the metal and serves as a source of potential (voltage) for the corrosion circuit. Since different metals require varying amounts of energy to refine, there are variations
in the amount of voltage available for the circuit. The following table from Patton5 is included to show the tendencies of metal to corrode. The half-cell potentials, which were reported by Peabody, were measured with a hydrogen reference electrode in a solution of the metal salt.
Galvanic corrosion occurs when the dissimilar metals are coupled in an electrolyte. The attack is from current flow within the simple “battery” formed by the metals and the water. Metals that are widely separated in the previous galvanic series will show the highest level of corrosion. Coatings on the metal surface, such as iron carbonates, block the galvanic current and lessen corrosion.
In any steel, the important sources of galvanic cell potential difference are:8
1. The various states of heat treatment of the steel, such as:
a. weld metal deposits,
b. the junction of weld and base metal,
c. tubing end heat treatment prior to upset (joint) manufacture
2. Cold Work and residual stress that result in anodes.
The Corrosion Circuit
The corrosion circuit requires an anode (the site of corrosion on the metal), a cathode, a metal connection between the anode and the cathode, and an electrolyte (liquid) surrounding the anode and the cathode.
Chemical Reaction
In acid solutions (pH e 7), reduction of hydrogen ions to hydrogen gas can be the dominant reaction in the absence of H2S gas. In neutral or basic soluiions (pH 17), reduction of oxygen is the dominate reaction. When gases such as CO2 and H2S are present, the reactions are modified by the gases. Presence of CO2 in neutral solutions can cause direct reaction of bicarbonate or carbonate ion with the steel. This can deposit a beneficial protective films such as iron carbonate. H2S on the metal surface stops the formation of hydrogen gas from hydrogen ions and permits a large percentage of the cathodic hydrogen ions to enter the steeL7 This is the start of one of the hydrogen embrittlements; cracking in hard steels or blistering in soft steels. As the metal corrodes, it dissolves at the anode and enters the solution as ions. It is an oxidation reaction since the iron leaves in an state. The electrons flow toward the cathode, where hydrogen gas is evolved. The schematic of the corrosion cell is shown in Figure 6.1 .5 The anode reactions are:This reaction is for corrosion produced in neutral, agitated salt water.5 The actual location of the anode and cathode may vary with the inhomogeneities in the metal and attack may be localized or may occur over a very wide area. The rate of reaction is dependent upon many factors, including the salinity of the water, flow velocity, temperature, pH, metal alloy characteristics, and dissolved gases such as oxygen, carbon dioxide and hydrogen sulfide.
The overall corrosion process results in weight loss at the anode caused by the loss of iron and hydrogen embrittlement of high strength and highly stressed steels by penetration of the atomic hydrogen. The corrosion reaction is most severe where pits are formed. The intensity of the pitting is affected by the manufacturing, handling and production factors. In these areas, abnormalities such as large grains, poor heat treating, improper stress relief, mill marks, pipe wrench nicks, damage during running, and other factors contribute heavily to the location of electrochemical attack that causes pits. Endean summarized the common sources of metal corrosion as:*
1. Hydrogen sulfide - causes both pitting and general attack. The reaction product is a black, usually shiny mass and may be in the form of a hard scale or a finely divided solid dispersed in the water.
2. Carbon dioxide - attack is through pitting with brown or black reaction product. Pits produced in CO2 attack are frequently in a line and resemble a large cavity. The remainder of the pipe may be unaffected.
3. High concentration chloride brines with a pH of 6-7 produce shallow wide spread pitting attack similar to acids but much less severe.
4. HCI - mineral acid attack produces severe general pitting with frequent occurrence of deep channels and deep pits.
5. Sulfate reducing bacteria - SRBs produce a localized corrosion by trapping their own low pH waste product and protecting the corrosion from inhibitor contact. The location of the corrosion is usually under the bacteria colony.
6. Erosion damage - high velocity contact by fluids, gases containing mists and droplets, or fluids containing solids generates a smooth surface with frequent shallow channels, plateaus, and sharply defined transition areas, especially around the area of highest fluid velocity and directly across from perforation.
The amount of corrosion is often expressed as a mils (or thousandths of an inch) per year, MPY. This means of expressing corrosion is only usable when the corrosion rate is an even attack on the surface of the steel. Where pits occur, an MPY value is useless: generation of even a few deep pits can ruin a piece of equipment without loosing but the smallest fraction of a percent of the total metal mass. Rate of pit growth varies with the depth and size of the pit and the rate of penetration of the pit will actually increase with depth of the pit.2 As the pit is growing, the very bottom of the pit is the anode. This area becomes smaller with pit depth as the pit forms a V shape. The smaller bottom area looses metal at a faster rate to satisfy the current flow of the corrosion circuit. This is the reason for pin hole leaks in an
otherwise solid piece of equipment.
Acid Gases
The special case of production of hydrogen sulfide gas, H2S, carbon dioxide gas, CO2, or a mixture of the two is the area of acid gas technology.’O The corrosion produces one or more types of Hydrogen Embrittlement, HE, in the steel. Hydrogen embrittlement reduces the toughness of steel (a loss of ductility) and is most prevalent around existing defects (micro or macroscopic) in the steel. The steels most susceptible to hydrogen embrittlement problems are those with a yield strength of 80,000 psi, or greater (N-80 and higher alloys). In lower strength steels, hydrogen blistering is occasionally found. The corrosion caused by acid gasses is influenced by the pH and by pressure, temperature, the corrosionresistance of the metal and the passive corrosion films formed on the surface of the metal.
Several forms of hydrogen embrittlements, HE, have been described including stress corrosion cracking
and stress sulfide cracking.’&’’
All forms of hydrogen embrittlement are brittle failures of a metal at a stress level below its yield
strength as a result of their exposure to atomic hydrogen.’’ The atomic hydrogen is generated on
metal surfaces by corrosion rea~t ion. ’T~h e hydrogen is diffused into the metal and causes a reduction
in the ductility of the metal. Sour gas increases the corrosion of HE by:‘’ (1) low pH of fluids that
contain H2S, (2) sulfide causes a greater percentage of the hydrogen created at the surface to enter
the metal, and (3) the anodic portion of the corrosion reaction tends to be localized, which helps
cracks initiate. The result of these actions is extremely rapid failure of some metals in sour fluids.
HE is generally associated with high strength steel and is common with H2S wells. The factors controlling
HE are:*
1. Steel yield strength - steel with yield strengths of 90,000 psi or lower (C-90, N-80, L-80, C-75,
etc.) are usually less susceptible to hydrogen embrittlement.
2. Hardness - Maximum hardness should be a Rockwell “C” scale of 22 or lower (the hard steels
are much more prone to attack from HE).
3. Stress level - At low stresses HE failures are lessened. In HE susceptible steels there is a
threshold below which HE will not occur. This threshold value is lowered for higher strength
steels.
4. Internal stress - The internal stress, which includes stored tensile stress produced by welding,
bending or surface damage is a common initiator for corrosion.
5. Hydrogen concentration - The time to failure of any high strength steel is a function of the concentration
of hydrogen.
6. Temperature - HE failures usually do not occur above 150°F. (A special exception to this is the
case of stress corrosion cracking7)
Two special cases of HE are sulfide corrosion cracking and stress sulfide cracking. Sulfide corrosion
cracking, SCC, causes a brittle failure of metals by the action of localized corrosion and stress.lg SCC
is normally encountered near the bottom of wells and in hotter environments than other forms of HE.7
In sour gas systems, SCC causes failure of high strength steels, all types of stainless, and many low
alloy nickel-based alloys.lg SCC will also occur in production of hot brines (chloride rich). SCC is common
in stainless alloys and materials. Alloys containing about 8% nickel are the most ~usceptible.~
Alloys with over about 42% nickel are usually immune to SCC.7 These alloys include Inconel, Incoloy,
Monel and Hasteloy. Other immune alloys may include cobalt-cromium-nickel-molybedenum alloys,
nickel-free low alloys, and nickel-free martensitic stainless steels.
There is a relationship between the environment and the metal to cause SCC; only certain metals will
crack in given environments at the critical stress level. SCC is considered to be an anodic process in
which a crack is initiated (usually by HE) and reaction progresses inside the crack. The dissolution of
metal at the tip of the crack controls the process. The environment inside the crack may be very different
from that on the surface of the casing due to the large area of metal and the protected environment.
The pH of the produced fluids, for example, may be between 4 and 6, while inside the crack, pH
may be between 1 and 2 (highly acid) because of higher concentration of chloride ions, which
increase the local corrosion rate.lg The overall corrosion rate of a material that is undergoing SCC
may be low and outer appearance may be good. However, the detrimental SCC corrosion in the crack
occurs as the result of the localized, often unseen, attack.
Stress sulfide cracking, SSC, occurs in high strength (high hardness) steels exposed to sour gas production.
It is also known as hydrogen stress cracking and hydrogen embrittlement cracking.20 SSC is
cracking that results from hydrogen charging (large volume entrance of hydrogen) of high strength
and/or high hardness steels. Most SSC occurs at lower temperatures and is prevalent in the upper
parts of the well. It may accelerate during periods of shutin or cool down, requiring only a reduction in
temperature to become active. SSC is a form of hydrogen embrittlement and is a bulk alteration of the
metal surrounding the surface areas.
Most corrosion rates increase with an increase in temperature up to about 140 to 150°F. At this point,
several forms of corrosion are lessened and some corrosion inhibiting films begin to form. Although an
increase in temperature renders the steel more susceptible to attack by SCC, an increase in temperature
decreases the rate of stress sulfide cracking, SSC. At higher temperatures, the atomic hydrogen
that contributes to the initiation of the crack by embrittlement is able to diffuse out of the steel. Temperature
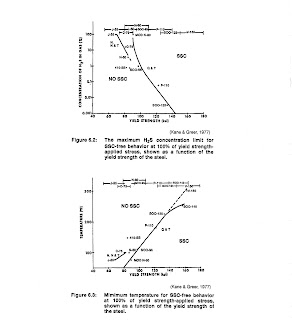
thresholds exist for SSC and above these limits, SSC does not occur. The limits for hydrogen
sulfide content and temperature are indicated in Figures 6.2 and 6-3. Figure 6.3 shows that the temperature
threshold for SSC free behavior is dependent upon the grade of steel. SSC can be controlled
with use of lower strength alloys.
Controlling Corrosion
Approaching corrosion control from a well completion position may involve selection of corrosionresistant
alloy,i1~i2*19*2’-24 films and coatings,2532 liquid
device^.'^^^-^^ The least expensive route will depend on the produced or injected fluids, completion
design and the level of protection required in the operation.
Modifying the produced fluid by changing pH or removing water or dissolved gasses such as oxygen,
CO2, or H2S are usually only available for use in pipelines and injection systems. Gas removal systems
such as gas stripping, degeneration and chemical treating may all be used to remove or reduce
the content of gases. Changing the character of the produced fluids is usually achieved by changing
operating conditions to control the separation of the condensing phase.
Coatings are a relatively simple and inexpensive way to isolate the metal (the anode and cathode)
from the electrolyte liquid. Permanent coatings include plastic, tars, cement and paint. Coatings are
usually chosen for a protection against a particular liquid. The plastic coatings, for example, include
resins and polymers that are resistant to low pH waters, oxygen, COs, or salts. Coatings are not resistant
to all influences however: acids, alcohols, and other materials will destroy some coatings. Care
must be taken in working over wells with coated tubing to avoid damage to the surface of the coating.
Abrasive action such as wireline action or coiled tubing are very detrimental. Damage to coated surfaces
offer sites for very localized, intense corrosion.
Liquid corrosion inhibitors act as temporary coatings or films on the surface and are effective in providing
a passive film or a coating if they are replenished on a regular basis. Selection and application
of inhibitors are critical elements in the corrosion control program of a well. There are literally hundreds
of chemical inhibitors for control of dozens of different corrosion problems on various types of
steels. The inhibitor for a particular application must be selected from lab or field tests at the conditions
where the corrosion will be active. Normally, these selection tests are started in the lab and completed
in the field with field trials on test metal coupons.
Complete reviews and comparisons of the methods of applying corrosion inhibitors are rare, but a few
case histories do exist. Houghton and We ~ t e rma r kh~av~e provided data on some corrosion problems
in the North Sea and compared the methods for application of corrosion inhibitors. In the wells that
were used for a database, average workover life was approximately 60-1 20 days. CO2 corrosion and
erosion were present in these wells’. Erosion was determined to have a significant influence on the
rate of corrosion and CO2 corrosion/erosion was found to be the normal mechanism of attack on these
wells.
During the study, the rate of corrosion for these wells was determined to be exponential rather than
linear. Once the corrosion started, very rapid increases in the corrosion rate were common. The most
prevalent place for attack of the corrosion/erosion was at changes in diameter or direction of the fluid
flow. The paper pointed out that sweet corrosion was prevalent in these wells even though there was
less than 14% formation water in the total produced fluids.
Ekofisk wells in the study that had a high GOR showed increased corrosion; probably by providing
greater volumes of CO2 and by increasing the flaw velocities of the produced fluids. The GOR has
also been shown to be a factor in corrosion in other studies. Even in gas wells, a change in flowing
fluid composition because of condensation of C-3+ hydrocarbons can result in a change in corrosion
intensity or location.40 The most common corrosion site depth was in the mid-range from 4000-
7000 ft. The mid-range location on these wells corresponds with gas breakout and increased turbulence
from suspended gas that is rapidly expanding due to the lowering of hydrostatic head.
In all cases of wells deviated more than 20°, a preferential attack along the low side of the tubing was
spotted. This attack reportedly resulted in troughs 1 in. wide that tracked along the inside of the low
side of the tubing.
This “pipe trough” development has also been though to be the result of a low oil wetting tendency of
the Ekofisk crude, which would result in a water wetted pipe. If the fluid velocity in these flowing wells
is below 2.5 to 3 ft/sec, the approximate minimum velocity for water entrainment in the oil, a free water
layer would exist at the lowest point due to gravity separation and increased corrosion could occur.8
The corrosion/erosion attack location was identified using casing calipers. Corrosion in caliper tracks
has also been observed. The cause may be that early caliper surveys were not followed with inhibitor
treatment to repair damaged protective films.
In the higher volume wells, preferential attack occurred on the pin-end shoulder on the coupling. The
shoulder seems to cause additional turbulence and pitting is a byproduct of the turbulence.
The paper reported a comparison of inhibitor treatment types, in terms of both economically application
and performance. Formation squeezes with inhibitor, continuous injection, and tubing displacements
were all examined. Continuous injection was found to be the least expensive in almost all flow
rates studied.
A second case study, and one that covers economics of chemical inhibitor usage was provided by
Akram and Butler.41 This work showed that the cost of the successful inhibitor protection program was
about $29,000 per well per year, compared with a super alloy tubular cost (for passive control) of
about 1.25 million. The cost of carbon steel tubulars for the same well was $271,000 (all dollar values
are 1982 U.S. dollars). The economic impact of the successful inhibitor program was significant;
34 years of inhibitor operation to equal the simple difference of super alloy and carbon steel tubular
cost. Obviously, the successful control of corrosion using either method depended upon good design
and strict application. All inhibitor films have to be replaced on a regular basis. While this addition is
relatively easy in injection wells by surface addition to injected fluids, it is more difficult in producing
wells. The inhibitor must be circulated into position and allowed to film on a clean surface without
being disturbed by action of other surfactants, inhibitors or solvents. Most inhibitors must be placed as
a dispersed phase in a non reactive fluid without the aid of surfactants. The application of these materials
may range from simple “dump” jobs down the back side (low pressure injection into the annulus
at the surface in a well without a packer) to periodic workovers requiring the well to be shutin while
inhibitor is injected down the tubing. Some wells are completed with a small string of tubing (1/4 in. to
1 in. diameter) down the outside or inside of the tubulars where the inhibitor and other treating chemicals
can be injected continuously.
Some naturally passive films (a reaction product of the metal and the wetting fluid) provide a barrier
surface that reduces the potential produced in the corrosion circuit by altering the reactivity of surface.
The film may be a metal oxide laye?5v27 or other reaction by-product that is not easily attacked by produced
fluid. These films are recognized as major corrosion controlling mechanisms. Corrosion of low
alloy steels at temperatures below 140°F, increase with the partial pressure of CO2 in the gas phase.
Above 140°F the corrosion decreases with temperature because of the formation of iron carbonate
and iron oxide films. The films are destroyed by acidizing or erosion during high velocity flow. In certain
cases, passivity is designed into the alloy by combining chromium and nickel with iron. Whether
these iron-chromium and iron-chromium-nickel alloys are active or passive depends upon the alloy
composition and the electrolyte. For example, in CO2 rich environments, 13% chrome alloys are successful
in preventing corrosion that destroys other alloys.25
Cathodic protection using sacrificial anodes or impressed current to offset the current of the corrosion
cell, can be applied to the outside of casing and pipelines and to the insides of production processing
vessels where a continuous water phase exists. It cannot be used internally in most production tubing
or inside pipelines.
Materials for Sour Service
The following description of materials for Sour Service is from Wilhelm and Kanelg and represents
generalized guidelines on selection of tubular components for hydrogen sulfide and carbon dioxide
service.
High strength tubular steel grades, often containing chromium and molybdenum designated for use in
H2S service include C75, L80, C90, and some specially processed C95. These materials exhibit necessary
resistance to SSC under some specific conditions for use in sour gas operations.
In general, the higher the yield strength of a material, the more susceptible it is to SSC. There are no
recognized carbon or low alloy steel compositions suitable for sour service at ambient temperatures
with yield strength in excess of 11 0,000 psi. The most widely used criterion for selection of materials
for sour service is hardness. NACE requirement MR-01-75 specifies that for steels to be considered,
they must have a hardness value below HRC-22 (some exceptions to HRC-26).4
Stainless steel casing (greater than 12% chromium) are used when superior resistance to general corrosion
is necessary. Table 2 shows composition of several of the high nickel alloy materials. The
steels increase in cost as corrosion resistance is increased.
The following paragraphs, also from Wilhelm and Kane,lg describe the general classifications of the
high strength alloys available for use in corrosive environments. Stainless steel is a generic term for a
group of steels having a chromium content of over 12%. Most metallurgists refer to the stainless steels
with the more widely based term “corrosion resistant alloy,” or CRA. The general classes of the alloys
are listed in order of increasing resistance to SCC and SSC (also increasing cost).
1.
2.
3.
4.
5.
6.
7.
Martensitic stainless steels, (11 -1 8% chromium) have applications in wellheads and tubing
where high yield strengths are not required.
Precipitation hardened stainless steels (12-1 8% chromium and 6-1 2% nickel) are useful for
downhole equipment or tools that require non-cold-worked, high yield strength materials. Some
of these materials, depending on composition, may be susceptible to SCC and SSC.
Duplex stainless steels (22-28% chromium and 5-7% nickel) have a resistance to chloride cracking
that exceeds the resistance of low alloy austenitic stainless steels, but they may be susceptible
to SSC or SCC in the presence of H2S.
Low alloy austentinic stainless steels (1 8% chromium and 10% nickel) offer better resistance to
SSC than martensitic stainless steels, but yield strengths are limited. These alloys are susceptible
to SCC and pitting by chlorides.
High alloy austentinic stainless steels contain 20-30% chromium and 20-35% nickel. They
achieve strength through cold work and offer the best combination of corrosion resistance and
mechanical properties of all the CRAs. The cost for these alloys is high.
Nickel-based super alloys such as C-276, 71 8, and MP35N (cobaltlnickel-based) have better
resistance to H2S than most other types of commercial alloys but may be extremely expensive.
They do have the advantage of very high yield strengths.
Titanium alloys are slowly being introduced to the industry although the use is rare at the present
time.
CO2 Corrosion
CO2, one of the acid gases, is a very common contaminate in gas, oil and water production, even in
sweet reservoirs. CO2 corrosion of steel is usually a localized corrosion that takes the form of pits of
various sizes. Liquid water is necessary for CO2 corrosion to take place.43 The typical corrosion product
of the CO2 reaction is ferrous carbonate.a Dissolved carbon dioxide content is a function of pressure
and temperature and pH is much less important. Corrosion increases for increasing carbon
dioxide content.
Pitting produces severe penetration. Outside of the affected areas, the corrosion rate might be limited
and the transition from an affected to an unaffected area can be very abrupt. The action of CO2 attack
has been described as both chemical and physical through e r o ~ i o nE.ro~s~ion~ c~an~ a ccelerate the
overall corrosion rate by a hundredfold or more by removal of protective scales, oxides and corrosion
inhibitor films. Even for CO2, however, the increase in rates usually is in the area of five to ten
The addition of CO2 gas to water can reduce the pH to a value below 4, promoting acid attack. CO2
corrosion from chemical attack has been generally effectively controlled through the use of 13%
chrome tubulars.
Although CO2 corrosion and stress sulfide cracking have nothing in common when both corrosion factors
are present in a well, control of both forms of corrosion may be accomplished by using a corrosion-
resistant alloy to block CO2 attack and a reduced hardness to prevent SSC. Choosing a 13%
chromium steel that has a hardness below 22 Rockwell hardness-C, (LSO tubing), should also be
effective.
One severe drawback to using the 13% chromium steels is that they exhibit very limited resistance to
pitting during storage where air and chloride are present (seacoasts). This type of corrosion can much
more significant inside the pipe, particularly if condensation inside the pipe forms standing puddles.24
In the well, control of pitting corrosion of the 13% chrome steels relies on the deaeration of water.
The second type of severe corrosional effect produced by CO2 is largely physical -the erosional effect
produced by changes in fluid flow direction or an effect often described as ~ a v i t a t i o n . ~E~ro*si~on~ is* ~ ~
the increase in the rate of metal deterioration from the abrasive effects of a fluid flowing into or
through a pipe. Other sources of erosion may include entrained gas in liquids, liquid droplets in gas,
solids in any fluid, very high flow rates, or any restriction in the completion strings that causes a drastic
change in the flow velocity of the produced fluids. Erosion may often lead to a removal of the effective
inhibitor, corrosion film, or reactant film. Severe cases can be identified by grooves or rounded
pits or holes that are usually smooth and lie along the direction of flow. Removal of a protective inhibitor
or corrosion oxide film takes place when the strain on the film or corrosion oxide layer exceeds the
strain for the failure of the film. Erosion by solids and droplets may also affect the tenacity of the film
on an exposed surface. The failure of a corrosion oxide layer takes considerably longer than the failure
for most film-forming materials.
Squeezing or continuous injection of inhibitors may not be cost effective if the completion string ID is
smaller than the minimum ID required to prevent erosion. If this principle is violated, the film may be
continuously stripped off, even at high loading rates. Not all inhibitor films react in the same manner to
stripping by erosion; a few products offer very good performance in high velocity applications.
Other Factors
The presence of oxygen either as dissolved or entrained gas substantially increases the corrosion,
Figure 6.4.2 In almost all fluid handling systems, oxygen must be removed prior to injection of water or
shipment of the fluids by pipeline.
The pH of the water and the velocity of the water influence the corrosion attack. The chart in
Figure 6.5 illustrates the relative corrosion of flowing and nonflowing fluids versus the fluid pH. The
velocities shown in the graph are for water velocities of 3 to 7 ft per second. Below 3 FPS, corrosion is
reduced at pHs above 7. Notice for the flowing case that there is very little corrosion in waters with a
pH above 7 (basic). In these high pH waters, iron is almost insoluble, so the byproducts of a corrosion
reaction cannot be swept away from the corrosion site and the corrosion reaction is stopped.
The amount of water in the production is also a factor in corrosion. Water cuts below 25% are likely to
cause less corrosion than water cuts above 45%, especially at pHs below 7. As water cut increases,
the tendency for a steel surface to be oil wet decreases. Corrosion is very low for most oil wet surfaces
and very high for most water wet surfaces. Since most wells are at least very slightly deviated,
the water caused corrosion damage may be confined to a trench in the low side of the pipe. In wells
where the flow rate in the tubing is not high enough to keep all the liquids moving at near the same
velocity, the water may reflux (up and down with gas rate changes) in the low side, creating a serious
corrosion trench even at very low water cuts. Inspection with caliper or electromagnetic surveys (measures
metal loss through field generation and interruption techniques) can usually spot the trench if
one exists. Water can be produced and carried as an emulsion (entrained water) at flow velocities of
3-1/2 to 5 fps.
Corrosion by Stimulation Acids
The use of stimulation acids such as HCI and HCVHF create severe problems in the specialty tubulars.
Special problems of selective effects of inhibitor^,^^ pitting and intergranular attack,47 detrimental
influence of added organics,48i49 and increased corrosion of the fluoride ion,50 require special inhibitors
and special inhibition techniques for the high allow steels. The primary attack of the steels by acid
is severe pitting and intergranular attack that is difficult to control with inhibitors. Localized pitting may
be severe enough to ruin a section of the string.
Acid inhibitors work in the same manner as other corrosion inhibitors; by filming and passivating the
surface. The HCI and HCVHF acids are much more severe environments than other types of corrosion
and the inhibitors for these uses are specially blended and have much shorter effective life spans.
Also, presence of mutual solvents, alcohols,
destroy the effectiveness of some inhibitors.
oil solvents and surfactants in the acid may alter or
Some of the expensive super alloys, such as the 13-chrome materials, may be especially sensitive to
HCVHF acid attack and special inhibitors are required.
Destruction of Elastomers
The steels used in the tubulars and well equipment are not the only materials susceptible to corrosion.
The elastomers (plastics and rubbers) used in the seals are also affected by the well fluids and must
be carefully selected to avoid problems. The attack on elastomers by gas is usually by swelling or blistering,
5’ both involve invasion of the elastomer by the gas. Rapid release of the pressure around a
gas permeated seal will likely cause explosive decompression and destruction of at least the outer
layer, the sealing edge, of the seal. Surprisingly, although gas permeation of a seal can destroy the
seal when pressure is released, the seal may function adequately before pressure release. Reaction
of various liquids to elastomer seals depends on seal type and position, temperature, liquid type, pressure,
previous seal contamination and the presence of some lubricants used for tool assembly.52 In
general, nitrile seals are used for most general purpose applications where oil contact is necessary.
Other compounds such as the fluorinated elastomers (e.g., Viton) are available for contact with aromatic
solvents (xylene and toluene). Other specialty compounds are available for specific, highly corrosive
conditions or contact with powerful solvents; however, seal cost increases quickly with the more
exotic elastomer compounds. Some metal-to-metal seals are being offered where elastomer destruction
is most severe.53
Microbial Corrosion
Microbiologically influenced corrosion (MIC) active corrosive influence of the attached (sessile) bacteria
c o l o n i e ~ . ’T~h*e~ p~ro blems are two fold; the colonies cover areas of steel, blocking corrosion
inhibitors from reaching the pipe surface, and the waste products of the colonies are often very corrosive
in the protected areas under a colony. The sulfate reducing bacteria, SRBs, are the most detrimental,
with the capability of souring wellbores and parts of reservoirs with H2S. Bacteria are
controlled by cleaning the water and treating with bactericides.
Nonmetallic Tubulars
As an alternative to steel casing with its problems with corrosion, fiberglass casing, tubing and rods
are being applied in some ~ e l l sTh. e~ ad~va~nta~ge~s o f the plastic materials are excellent resistance
to most forms of water related corrosion and some scale and paraffin deposition. Most applications
have been in shallow, low pressure wells, where high strength is not needed; however, new placement
techniques and plastic formulations are stretching limits of application.
Predictive Techniques and Inspection Devices
Monitoring the rate of corrosion is of critical importance to determine when to repair or replace equipment
and to judge the effectiveness of corrosion control techniques on well e q ~ i p m e n t .A~ s~y-s~te~m -
atic examination of the produced fluid chemistry, operating conditions and failures can describe the
potential for further failure.55 Monitoring of corrosion in the well uses several logging techniques to
monitor abrasion (and other wear), pitting and surface corrosion.
The data from produced fluid analysis includes ion analysis, pH, gas type and content. It can be used
with computed based predictive models to establish a general potential for corrosion in the well. Corrosion
coupons inserted into the well stream can verify the predictive results and help evaluate the
effect of an inhibitor. Sections of test pipe are also used, normally in surface piping, to evaluate
selected metals.
When a corrosion related failure occurs, it is most important that the cause of the failure be determined.
The analysis of corrosion products and the characteristic corrosion pattern can usually determine
the type of corrosion that caused the failure. Hardness tests, microscopic examination, and
chemical analysis of the failed and unaffected surfaces are also tools of identifi~ation.~~
To monitor the corrosion rate and general condition of the tubulars in the well, a set of instruments are
used that provide data for comparisons with earlier readings to arrive at a corrosion or erosion rate.
These tools include multifingered c a l i p e r ~p,r~ob~e s for measuring anodic activity,57 induction tools to
measure pipe mass,58 magnetic devices,59 sonic tools that measure pipe thickness,60 and some
experimental tools that locate cracks in the pipe.60 These tools will establish a rate of corrosion or
wear when the results from several regular runs are compared.
Erosion
Surface erosion from solid particles in a high velocity produced fluid stream is normally associated
with unstable formations such as unconsolidated sands. Other occurrences of erosion include cases
of choke and tree loss following rapid back flow of wells after fracturing. The common denominator is
the high flow velocity.
Maximum velocities that can be tolerated in a situation will depend on the flowing fluid and other factors
including foaming or emulsifying tendency, solids and entrained gas. Mechanical limitations in the
piping design or metering apparatus may also influence the maximum permissible flow rate.
Although some information exists that a corrosion inhibitor film is removable by high velocity flow,2i61
other authors offer evidence of successful inhibitor film performance at mass velocities of up to
100 Wsec with abrasion where inhibitor was continuously present.62 In any design where flow rates
will be high, a testing program should be used to identify the best method of corrosion protection.
The concept of critical velocity for flow of fluids with no solids in sizing of piping is covered in API RP-
14E.63 In general, the limits for dry crude flow velocity in pipe is about 30 to 35 fps (ft per second) and
for wet crude, the maximum velocity is 20 to 25 fps. At faster flow rates, some steel may be lost to
abrasion from the clean liquids. In some inhibitor protected systems, the limit of fluid velocity is often
much higher than set by the API equation.
The erosion rate of any metal surface is strongly controlled by the presence and hardness of naturally
occurring (but metal-composition related) oxide or sulfide film. This film, which depending on composition,
can be much harder than the pure metal surface, is one of the main factors that reduce erosion
(and some forms of chemical corrosion).
Presence of mist droplets in the stream can destroy the natural or added inhibitor barriers and rapidly
increase corrosion. Actual abrasive induced failures of the pipe depend on the entrained droplets or
solids in the gas as well as the density of the gas. Estimates of the densities and good design velocities
are contained in Figure 6.6. For further information, refer to the “Oil Field Corrosion Detection and
Control Handbook,” by Endean, available from Champion Chemicals Inc., Houston.
The API RP-14E equation is a quasi-rigorous attempt to determine critical velocity for general purpose
projects. Craig 68 proposed flow ranges for a modification of the RP-14E equation, proposed by Griffith
and Rabinowicx (1985), where the C factor was calculated. The calculated value of C was based
on actual well conditions. The equation was only very slightly different:
but the C was dependent on flowing fluid and pipe metallurgy, rather than a range of operating constants.
When using chemical resistant alloys such as stainless and some nickel based materials
(especially those containing Chromium), the stable range of the C factor in the equation would be in
the range of 160 to 300. The 160 to 300 range for CRA tubulars is well proven infield performance for
long lived projects.
The ultimate value of C is then dependent on alloy composition, oxide or sulfide layer composition,
and flowing fluid composition (H2S, CO2, etc.). The layer composition and fluid resistance would also
change with temperature and impact resistance. Craig proposed a further modification of the equation
to allow input of the oxide hardness layer
where P is the measured hardness of the oxide layer in kg/mm2 and p is fluid density in Ib/ft3. The use
of such an equation for calculations is limited at the present time by available data on hardness, P.
Craig offers the following explanation: “for Ti alloys, using a hardness of 1,000 kglmm2 for titanium
dioxide (Ti02) film formed on the alloy, the C factor would be 189. However, if aluminum oxide (aI2O3)
were incorporated into the oxide of any alloy so it was the predominate film, then P E 200 kg/mm2 and
C = 255. Conversely, if a SS or Ni-based alloy containing Cr is exposed to a high H2S environment,
then the film could be predominately Cr2S3, with P - 480 kg/mm2 and C would drop to 138l (with data
from A.A. Ivan’ko - Handbook of Hardness),
Impingement of particle laden fluids on a screen, casing wall, choke, or other surface in the well will
result in some erosion, regardless of the velocity flow. Erosion is usually only severe however, when
the fluid flow velocity is high enough to impact the solid particles on the surface with enough force to
abrade the metal or the natural or man-made coating on the metal surface.
Well completion decisions in wells with solids erosion problems may take one or more of three routines:
(1) decrease the flowing liquid velocity with larger perforations, larger wellbores and larger tubing,
(2) use hardened blast joints to slow the rate of erosion, and (3) control the solids with gravel
packs and screens.
If the erosion problem is slight, blast joints represent the cheapest methods of control. If erosion is
severe, the producing pays are usually gravel packed.
The erosion rate of any surface exposed to fluids that contain solids depends on the size, roundness,
and composition of the solids, the amount of solids in the flow stream, the velocity of the flowing fluid,
the shape of the flow path and the hardness of the metal layer exposed to the fluid. The worst erosion
conditions are created when sand grain sized (0.01 to O.l”), particles of high density materials rapidly
strike the metal surface at angles of about 45” to 95”. If the energy of the impact is sufficient to dislodge
or break the oxide layer, then erosion and some forms of corrosion will be accelerated.